Rapid microbial viability assay using scanning electron microscopy: a proof-of-concept using Phosphotungstic acid staining
- PMID: 37501704
- PMCID: PMC10371768
- DOI: 10.1016/j.csbj.2023.07.010
Rapid microbial viability assay using scanning electron microscopy: a proof-of-concept using Phosphotungstic acid staining
Abstract
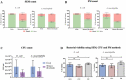
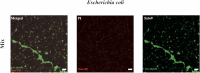
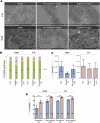
Multiple stains have been historically utilized in electron microscopy to provide proper contrast and superior image quality enabling the discovery of ultrastructures. However, the use of these stains in microbiological viability assessment has been limited. Phosphotungstic acid (PTA) staining is a common negative stain used in scanning electron microscopy (SEM). Here, we investigate the feasibility of a new SEM-PTA assay, aiming to determine both viable and dead microbes. The optimal sample preparation was established by staining bacteria with different PTA concentrations and incubation times. Once the assay conditions were set, we applied the protocol to various samples, evaluating bacterial viability under different conditions, and comparing SEM-PTA results to culture. The five minutes 10% PTA staining exhibited a strong distinction between viable micro-organisms perceived as hypo-dense, and dead micro-organisms displaying intense internal staining which was confirmed by high Tungsten (W) peak on the EDX spectra. SEM-PTA viability count after freezing, freeze-drying, or oxygen exposure, were concordant with culture. To our knowledge, this study is the first contribution towards PTA staining of live and dead bacteria. The SEM-PTA strategy demonstrated the feasibility of a rapid, cost-effective and efficient viability assay, presenting an open-view of the sample, and providing a potentially valuable tool for applications in microbiome investigations and antimicrobial susceptibility testing.
Keywords: Fluorescent microscopy; Phosphotungstic acid; Plate count; Scanning electron microscopy; Viability.
© 2023 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Authors would like to declare that D.R. was a consultant in microbiology for the Hitachi High-Tech Corporation from March 2018 until March 2021. Y.O. is employed by the company Hitachi High-Tech Corporation. A.H. is employed by the company Hitachi, Ltd. Personal fees of G.H., S.B., and J.B.K. are paid through a collaborative contract from the company Hitachi High-Tech Corporation. O.Z. declares no relevant competing interest. This work was supported by a grant from the French Government managed by the National Research Agency under the “Investissements d’avenir (Investments for the Future)” program with the reference ANR-10-IAHU-03 (Méditerranée Infection), by the Région Provence-Alpes-Côte-d’Azur and the European funding FEDER PRIMI. In addition, collaborative study conducted by IHU Méditerranée Infection and the Hitachi High-Tech Corporation is funded by the Hitachi High-Tech Corporation.
Figures





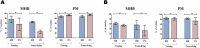


References
-
- Costello J., Blaize J., L’Amoreaux W., McCoy E. Ultrastructure of the pathogenic bacteria mobiluncus mulieris. MAM. 2007:13. doi: 10.1017/S1431927607076258. - DOI
LinkOut - more resources
Full Text Sources
