Vitronectin Destroyed Intestinal Epithelial Cell Differentiation through Activation of PDE4-Mediated Ferroptosis in Inflammatory Bowel Disease
- PMID: 37501933
- PMCID: PMC10371469
- DOI: 10.1155/2023/6623329
Vitronectin Destroyed Intestinal Epithelial Cell Differentiation through Activation of PDE4-Mediated Ferroptosis in Inflammatory Bowel Disease
Abstract
Objective: Vitronectin (VTN) has been reported to trigger cell pyroptosis to aggravate inflammation in our previous study. However, the function of VTN in inflammatory bowel disease (IBD) remains to be addressed.
Methods: Real-time PCR and western blotting were performed to analyze VTN-regulated intestinal epithelial cell (IEC) differentiation through ferroptosis, and immunofluorescence (IF), luciferase, and chromatin immunoprecipitation were used to identify whether VTN-modulated ferroptosis is dependent on phosphodiesterase 4 (PDE4)/protein kinase A (PKA)/cyclic adenosine monophosphate-response element-binding protein (CREB) cascade pathway. In vivo experiment in mice and a pilot study in patients with IBD were used to confirm inhibition of PDE4-alleviated IECs ferroptosis, leading to cell differentiation during mucosal healing.
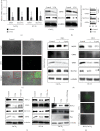
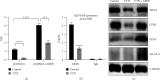
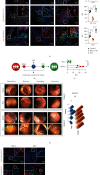
Results: Herein, we found that caudal-related homeobox transcription factor 2-mediated IECs differentiation was impaired in response to VTN, which was attributed to enhanced ferroptosis characterized by decreased glutathione peroxidase 4 (GPX4) and solute carrier family 7 member 11 expression. Inhibition of ferroptosis in IECs rescued the inhibitory effect of VTN on cell differentiation. Further analysis showed that VTN triggered phosphorylation of PDE4, leading to inhibit PKA/CREB activation and CREB nuclear translocation, which further reduced GPX4 transactivation. Endogenous PKA interacted with CREB, and this interaction was destroyed in response to VTN stimulation. What is more, overexpression of CREB in CaCO2 cells overcame the promotion of VTN on ferroptosis. Most importantly, inhibition of PDE4 by roflumilast or dipyridamole could alleviate dextran sulfate sodium-induced colitis in mice and in a pilot clinical study confirmed by IF.
Conclusions: These findings demonstrated that highly expressed VTN disrupted IECs differentiation through PDE4-mediated ferroptosis in IBD, suggesting targeting PDE4 could be a promising therapeutic strategy for patients with IBD.
Copyright © 2023 Wenxu Pan et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
-
- Huang L., Tang X., Yang F. Y., et al. Shikonin contributes to intestinal epithelial cell differentiation through PKM2/NRF2-mediated polyol pathway. Pharmacological Research – Modern Chinese Medicine . 2021;1 doi: 10.1016/j.prmcm.2021.100004.100004 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

