Stabilization of enzyme-immobilized hydrogels for extended hypoxic cell culture
- PMID: 37502125
- PMCID: PMC10373429
- DOI: 10.1007/s42247-019-00038-4
Stabilization of enzyme-immobilized hydrogels for extended hypoxic cell culture
Abstract
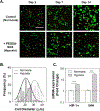
In this work, glucose oxidase (GOx)-immobilized hydrogels are developed and optimized as an easy and convenient means for creating solution hypoxia in a regular incubator. Specifically, acrylated GOx co-polymerizes with poly(ethylene glycol) diacrylate (PEGDA) to form PEGDA-GOx hydrogels. Results show that freeze-drying and reaction by-products, hydrogen peroxide, negatively affect oxygen-consuming activity of network-immobilized GOx. However, the negative effects of freeze-drying can be mitigated by addition of trehalose/raffinose in the hydrogel precursor solution, whereas the inhibition of GOx caused by hydrogen peroxide can be prevented via addition of glutathione (GSH) in the buffer/media. The ability to preserve enzyme activity following freeze-drying and during long-term incubation permits facile application of this material to induce long-term solution/media hypoxia in cell culture plasticware placed in a regular CO2 incubator.
Keywords: Hypoxia; cancer; enzyme immobilization; glucose oxidase; hydrogel.
Conflict of interest statement
Conflict of interest statement The authors declare no conflict of interest.
Figures

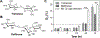

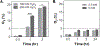

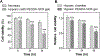
References
-
- Hockel M & Vaupel P Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst 2001. (93), 266–276. - PubMed
-
- Liu L & Simon MC Regulation of transcription and translation by hypoxia. Cancer Biol Ther 2004. (3), 492–497. - PubMed
-
- Huang Y, Zitta K, Bein B, Steinfath M & Albrecht M An insert-based enzymatic cell culture system to rapidly and reversibly induce hypoxia: investigations of hypoxia-induced cell damage, protein expression and phosphorylation in neuronal IMR-32 cells. Dis Model Mech 2013. (6), 1507–1514. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
