Plasma TRAIL and ANXA1 in diagnosis and prognostication of pulmonary arterial hypertension
- PMID: 37502145
- PMCID: PMC10368940
- DOI: 10.1002/pul2.12269
Plasma TRAIL and ANXA1 in diagnosis and prognostication of pulmonary arterial hypertension
Abstract
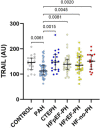
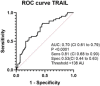
Pulmonary arterial hypertension (PAH) is a rare vasculopathy, with high morbidity and mortality. The sensitivity of the current european society of cardiology/european respiratory society (ESC/ERS) risk assessment strategy may be improved by the addition of biomarkers related to PAH pathophysiology. Such plasma-borne biomarkers may also reduce time to diagnosis, if used as diagnostic tools in patients with unclear dyspnea, and in guiding treatment decisions. Plasma levels of proteins related to tumor necrosis factor (TNF), inflammation, and immunomodulation were analyzed with proximity extension assays in patients with PAH (n = 48), chronic thromboembolic pulmonary hypertension (PH; CTEPH, n = 20), PH due to left heart failure (HF) with preserved (HFpEF-PH, n = 33), or reduced (HFrEF-PH, n = 36) ejection fraction, HF without PH (n = 15), and healthy controls (n = 20). TNF-related apoptosis-inducing ligand (TRAIL) were lower in PAH versus the other disease groups and controls (p < 0.0082). In receiver operating characteristics analysis, TRAIL levels identified PAH from the other disease groups with a sensitivity of 0.81 and a specificity of 0.53 [area under the curve: 0.70; (95% confidence interval, CI: 0.61-0.79; p < 0.0001)]. In both single (p < 0.05) and multivariable Cox regression models Annexin A1 (ANXA1) [hazard ratio, HR: 1.0367; (95% CI: 1.0059-1.0684; p = 0.044)] and carcinoembryonic antigen-related cell adhesion molecule 8 [HR: 1.0603; (95% CI: 1.0004-1.1237; p = 0.0483)] were significant predictors of survival, adjusted for age, female sex and ESC/ERS-initial risk score. Low plasma TRAIL predicted PAH among patients with dyspnea and differentiated PAH from those with CTEPH, HF with and without PH; and healthy controls. Higher plasma ANXA1 was associated with worse survival in PAH. Larger multicenter studies are encouraged to validate our findings.
Keywords: TNF‐signaling; biomarkers; immunomodulation; inflammation; pulmonary hypertension.
© 2023 The Authors. Pulmonary Circulation published by John Wiley & Sons Ltd on behalf of Pulmonary Vascular Research Institute.
Conflict of interest statement
Dr. Roger Hesselstrand is also an employee at Boehringer Ingelheim since October 2021. Dr. Abdulla Ahmed and Dr. Salaheldin Ahmed report personal lecture fees from Janssen‐Cilag AB and Nordic Infucare, outside the submitted work. Dr. Göran Rådegran reports personal lecture fees from Actelion Pharmaceuticals, Bayer Health Care, GlaxoSmithKline, Janssen Cilag AB, and Nordic Infucare, outside the submitted work. Dr. Göran Rådegran has received unrestricted research grants from Actelion Pharmaceuticals, GlaxoSmithKline, Nordic Infucare, and a noninterventional investigator‐initiated study research grant from Janssen Cilag AB. Göran Rådegran is and has been primary‐, or co‐, investigator in; clinical PAH trials for Acceleron, Actelion Pharmaceuticals, Bayer, GlaxoSmithKline, Janssen, Pfizer, and United Therapeutics, and in clinical heart transplantation immunosuppression trials for Novartis. The remaining authors declare no conflict of interest.
Figures

References
-
- Khou V, Anderson JJ, Strange G, Corrigan C, Collins N, Celermajer DS, Dwyer N, Feenstra J, Horrigan M, Keating D, Kotlyar E, Lavender M, McWilliams TJ, Steele P, Weintraub R, Whitford H, Whyte K, Williams TJ, Wrobel JP, Keogh A, Lau EM. Diagnostic delay in pulmonary arterial hypertension: insights from the Australian and New Zealand pulmonary hypertension registry. Respirology. 2020;25:863–871. 10.1111/resp.13768 - DOI - PubMed
-
- Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, ESC Scientific Document G. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67–119. 10.1093/eurheartj/ehv317 - DOI - PubMed
-
- Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger R, Brida M, Carlsen J, Coats A, Escribano‐Subias P, Ferrari P, Ferreira DS, Ghofrani HA, Giannakoulas G, Kiely DG, Mayer E, Meszaros G, Nagavci B, Olsson KM, Pepke‐Zaba J, Quint JK, Rådegran G, Simonneau G, Sitbon O, Tonia T, Toshner M, Vachiery JL, Vonk Noordegraaf A, Delcroix M, Rosenkranz S, ESC/ERS Scientific Document G. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618–3731. 10.1093/eurheartj/ehac237 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

