Protective efficacy of Toxoplasma gondii GRA12 or GRA7 recombinant proteins encapsulated in PLGA nanoparticles against acute Toxoplasma gondii infection in mice
- PMID: 37502604
- PMCID: PMC10368986
- DOI: 10.3389/fcimb.2023.1209755
Protective efficacy of Toxoplasma gondii GRA12 or GRA7 recombinant proteins encapsulated in PLGA nanoparticles against acute Toxoplasma gondii infection in mice
Abstract
Background: Toxoplasma gondii is an apicomplexan parasite that affects the health of humans and livestock, and an effective vaccine is urgently required. Nanoparticles can modulate and improve cellular and humoral immune responses.
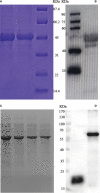
Methods: In the current study, poly (D, L-lactic-co-glycolic acid) (PLGA) nanoparticles were used as a delivery system for the T. gondii dense granule antigens GRA12 and GRA7. BALB/c mice were injected with the vaccines and protective efficacy was evaluated.
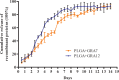
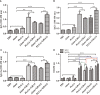
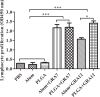
Results: Mice immunized with PLGA+GRA12 exhibited significantly higher IgG, and a noticeable predominance of IgG2a over IgG1 was also observed. There was a 1.5-fold higher level of lymphocyte proliferation in PLGA+GRA12-injected mice compared to Alum+GRA12-immunized mice. Higher levels of IFN-g and IL-10 and a lower level of IL-4 were detected, indicating that Th1 and Th2 immune responses were induced but the predominant response was Th1. There were no significant differences between Alum+GRA7-immunized and PLGA+GRA7-immunized groups. Immunization with these four vaccines resulted in significantly reduced parasite loads, but they were lowest in PLGA+GRA12-immunized mice. The survival times of mice immunized with PLGA+GRA12 were also significantly longer than those of mice in the other vaccinated groups.
Conclusion: The current study indicated that T. gondii GRA12 recombinant protein encapsulated in PLGA nanoparticles is a promising vaccine against acute toxoplasmosis, but PLGA is almost useless for enhancing the immune response induced by T. gondii GRA7 recombinant protein.
Keywords: GRA12; GRA7; PLGA; Toxoplasma gondii; nanoparticles; vaccine.
Copyright © 2023 Sun, Deng, Fu, Deng, Xie, Huang, Qi and Shi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Bacillus subtilis spores displaying Toxoplasma gondii GRA12 induce immunity against acute toxoplasmosis.Front Immunol. 2025 Feb 26;16:1457560. doi: 10.3389/fimmu.2025.1457560. eCollection 2025. Front Immunol. 2025. PMID: 40079011 Free PMC article.
-
Enhancing Immune Responses to a DNA Vaccine Encoding Toxoplasma gondii GRA7 Using Calcium Phosphate Nanoparticles as an Adjuvant.Front Cell Infect Microbiol. 2021 Dec 16;11:787635. doi: 10.3389/fcimb.2021.787635. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34976863 Free PMC article.
-
Protective immunity induced with a DNA vaccine encoding B- and T-cells multi-epitope SAG1, ROP16, MIC4, GRA12, M2AP, and multi-epitope ROP8 against acute and chronic toxoplasmosis in BALB/c mice.Exp Parasitol. 2022 Nov;242:108385. doi: 10.1016/j.exppara.2022.108385. Epub 2022 Sep 24. Exp Parasitol. 2022. PMID: 36162598
-
PLGA Nanoparticles as an Efficient Platform in Protein Vaccines Against Toxoplasma gondii.Acta Parasitol. 2022 Jun;67(2):582-591. doi: 10.1007/s11686-021-00499-w. Epub 2022 Jan 11. Acta Parasitol. 2022. PMID: 35013939 Review.
-
A systematic review on the role of GRA proteins of Toxoplasma gondii in host immunization.J Microbiol Methods. 2019 Oct;165:105696. doi: 10.1016/j.mimet.2019.105696. Epub 2019 Aug 20. J Microbiol Methods. 2019. PMID: 31442457
Cited by
-
Live-attenuated PruΔgra72 strain of Toxoplasma gondii induces strong protective immunity against acute and chronic toxoplasmosis in mice.Parasit Vectors. 2024 Sep 5;17(1):377. doi: 10.1186/s13071-024-06461-9. Parasit Vectors. 2024. PMID: 39237959 Free PMC article.
-
Bacillus subtilis spores displaying Toxoplasma gondii GRA12 induce immunity against acute toxoplasmosis.Front Immunol. 2025 Feb 26;16:1457560. doi: 10.3389/fimmu.2025.1457560. eCollection 2025. Front Immunol. 2025. PMID: 40079011 Free PMC article.
-
Design and immunological evaluation of a multi-epitope vaccine candidate against Toxoplasma gondii incorporating MIC13, GRA1, and SAG1 antigens in BALB/c mice.Food Waterborne Parasitol. 2025 Jun 2;40:e00269. doi: 10.1016/j.fawpar.2025.e00269. eCollection 2025 Sep. Food Waterborne Parasitol. 2025. PMID: 40546389 Free PMC article.
References
-
- Ahmadpour E., Sarvi S., Hashemi Soteh M. B., Sharif M., Rahimi M. T., Valadan R., et al. . (2017). Enhancing immune responses to a DNA vaccine encoding Toxoplasma gondii GRA14 by calcium phosphate nanoparticles as an adjuvant. Immunol. Lett. 185, 40–47. doi: 10.1016/j.imlet.2017.03.006 - DOI - PubMed
-
- Allahyari M., Mohit E., Amiri S., Esmaeili Rastaghi A. R., Babaie J., Mahdavi M., et al. . (2016). Synergistic effect of rSAG1 and rGRA2 antigens formulated in PLGA microspheres in eliciting immune protection against Toxoplasama gondii . Exp. Parasitol. 170, 236–246. doi: 10.1016/j.exppara.2016.09.008 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

