Phosphatidylserine decarboxylase downregulation in uric acid‑induced hepatic mitochondrial dysfunction and apoptosis
- PMID: 37502610
- PMCID: PMC10369160
- DOI: 10.1002/mco2.336
Phosphatidylserine decarboxylase downregulation in uric acid‑induced hepatic mitochondrial dysfunction and apoptosis
Abstract
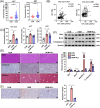
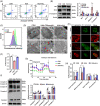
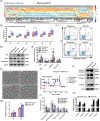
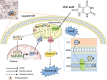
The molecular mechanisms underlying uric acid (UA)-induced mitochondrial dysfunction and apoptosis have not yet been elucidated. Herein, we investigated underlying mechanisms of UA in the development of mitochondrial dysfunction and apoptosis. We analyzed blood samples of individuals with normal UA levels and patients with hyperuricemia. Results showed that patients with hyperuricemia had significantly elevated levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels, which may indicate liver or mitochondrial damage in patients with hyperuricemia. Subsequently, lipidomic analysis of mouse liver tissue mitochondria and human liver L02 cell mitochondria was performed. Compared with control group levels, high UA increased mitochondrial phosphatidylserine (PS) and decreased mitochondrial phosphatidylethanolamine (PE) levels, whereas the expression of mitochondrial phosphatidylserine decarboxylase (PISD) that mediates PS and PE conversion was downregulated. High UA levels also inhibited signal transducer and activator of transcription 3 (STAT3) phosphorylation as well as mitochondrial respiration, while inducing apoptosis both in vivo and in vitro. Treatment with allopurinol, overexpression of PISD, and lyso-PE (LPE) administration significantly attenuated the three above-described effects in vitro. In conclusion, UA may induce mitochondrial dysfunction and apoptosis through mitochondrial PISD downregulation. This study provides a new perspective on liver damage caused by hyperuricemia.
Keywords: STAT3; apoptosis; lipidomics; mitochondrial dysfunction; phosphatidylserine decarboxylase.
© 2023 The Authors. MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no competing interest.
Figures



Similar articles
-
Fuling-Zexie formula attenuates hyperuricemia-induced nephropathy and inhibits JAK2/STAT3 signaling and NLRP3 inflammasome activation in mice.J Ethnopharmacol. 2024 Jan 30;319(Pt 2):117262. doi: 10.1016/j.jep.2023.117262. Epub 2023 Oct 1. J Ethnopharmacol. 2024. PMID: 37788785
-
Phosphoethanolamine cytidylyltransferase ameliorates mitochondrial function and apoptosis in hepatocytes in T2DM in vitro.J Lipid Res. 2023 Mar;64(3):100337. doi: 10.1016/j.jlr.2023.100337. Epub 2023 Jan 28. J Lipid Res. 2023. PMID: 36716821 Free PMC article.
-
The impact of chrysanthemi indici flos-enriched flavonoid part on the model of hyperuricemia based on inhibiting synthesis and promoting excretion of uric acid.J Ethnopharmacol. 2024 Oct 28;333:118488. doi: 10.1016/j.jep.2024.118488. Epub 2024 Jun 24. J Ethnopharmacol. 2024. PMID: 38925319
-
Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
-
Uric acid-induced pancreatic β-cell dysfunction.BMC Endocr Disord. 2021 Feb 16;21(1):24. doi: 10.1186/s12902-021-00698-6. BMC Endocr Disord. 2021. PMID: 33593356 Free PMC article. Review.
Cited by
-
Integrated multi-omic analysis identifies fatty acid binding protein 4 as a biomarker and therapeutic target of ischemia-reperfusion injury in steatotic liver transplantation.Cell Mol Life Sci. 2024 Feb 10;81(1):83. doi: 10.1007/s00018-023-05110-1. Cell Mol Life Sci. 2024. PMID: 38341383 Free PMC article.
-
Anti-Cancer Potential of Isoflavone-Enriched Fraction from Traditional Thai Fermented Soybean against Hela Cervical Cancer Cells.Int J Mol Sci. 2024 Aug 27;25(17):9277. doi: 10.3390/ijms25179277. Int J Mol Sci. 2024. PMID: 39273231 Free PMC article.
-
Alleviating the Effects of Electrolyzed Alkaline Water on Hyperuricemia in Mice.Nutrients. 2025 May 14;17(10):1673. doi: 10.3390/nu17101673. Nutrients. 2025. PMID: 40431413 Free PMC article.
-
Phyllanthi Fructus ameliorates hyperuricemia and kidney injure via inhibiting uric acid synthesis, modulating urate transporters, and alleviating inflammation.Sci Rep. 2024 Nov 11;14(1):27605. doi: 10.1038/s41598-024-79350-x. Sci Rep. 2024. PMID: 39528682 Free PMC article.
-
Age-associated reduction in ER-Mitochondrial contacts impairs mitochondrial lipid metabolism and autophagosome formation in the heart.Cell Death Differ. 2025 Apr 20. doi: 10.1038/s41418-025-01511-w. Online ahead of print. Cell Death Differ. 2025. PMID: 40254645
References
-
- Battelli MG, Bortolotti M, Polito L, Bolognesi A. The role of xanthine oxidoreductase and uric acid in metabolic syndrome. Biochim Biophys Acta Mol Basis Dis. 2018;1864(8):2557‐2565. - PubMed
-
- Li C, Hsieh MC, Chang SJ. Metabolic syndrome, diabetes, and hyperuricemia. Curr Opin Rheumatol. 2013;25(2):210‐216. - PubMed
-
- Isaka Y, Takabatake Y, Takahashi A, Saitoh T, Yoshimori T. Hyperuricemia‐induced inflammasome and kidney diseases. Nephrol Dial Transplant. 2016;31(6):890‐896. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
