The transcriptional landscape of endogenous retroelements delineates esophageal adenocarcinoma subtypes
- PMID: 37502711
- PMCID: PMC10370457
- DOI: 10.1093/narcan/zcad040
The transcriptional landscape of endogenous retroelements delineates esophageal adenocarcinoma subtypes
Abstract
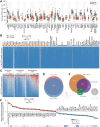
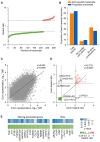
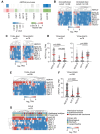
Most cancer types exhibit aberrant transcriptional activity, including derepression of retrotransposable elements (RTEs). However, the degree, specificity and potential consequences of RTE transcriptional activation may differ substantially among cancer types and subtypes. Representing one extreme of the spectrum, we characterize the transcriptional activity of RTEs in cohorts of esophageal adenocarcinoma (EAC) and its precursor Barrett's esophagus (BE) from the OCCAMS (Oesophageal Cancer Clinical and Molecular Stratification) consortium, and from TCGA (The Cancer Genome Atlas). We found exceptionally high RTE inclusion in the EAC transcriptome, driven primarily by transcription of genes incorporating intronic or adjacent RTEs, rather than by autonomous RTE transcription. Nevertheless, numerous chimeric transcripts straddling RTEs and genes, and transcripts from stand-alone RTEs, particularly KLF5- and SOX9-controlled HERVH proviruses, were overexpressed specifically in EAC. Notably, incomplete mRNA splicing and EAC-characteristic intronic RTE inclusion was mirrored by relative loss of the respective fully-spliced, functional mRNA isoforms, consistent with compromised cellular fitness. Defective RNA splicing was linked with strong transcriptional activation of a HERVH provirus on Chr Xp22.32 and defined EAC subtypes with distinct molecular features and prognosis. Our study defines distinguishable RTE transcriptional profiles of EAC, reflecting distinct underlying processes and prognosis, thus providing a framework for targeted studies.
© The Author(s) 2023. Published by Oxford University Press on behalf of NAR Cancer.
Figures






References
-
- Rebollo R., Romanish M.T., Mager D.L. Transposable elements: an abundant and natural source of regulatory sequences for host genes. Annu. Rev. Genet. 2012; 46:21–42. - PubMed
-
- Ishak C.A., De Carvalho D.D. Reactivation of endogenous retroelements in cancer development and therapy. Annu. Rev. Cancer. Biol. 2020; 4:159–176.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

