This is a preprint.
Selective Blood Cell Hitchhiking in Whole Blood with Ionic Liquid-Coated PLGA Nanoparticles to Redirect Biodistribution After Intravenous Injection
- PMID: 37502854
- PMCID: PMC10371090
- DOI: 10.21203/rs.3.rs-3146716/v1
Selective Blood Cell Hitchhiking in Whole Blood with Ionic Liquid-Coated PLGA Nanoparticles to Redirect Biodistribution After Intravenous Injection
Abstract
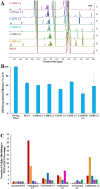
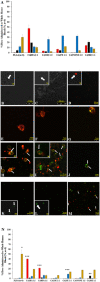
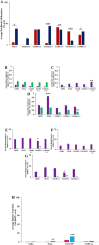
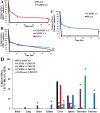
Less than 5% of intravenously-injected nanoparticles (NPs) reach destined sites in the body due to opsonization and immune-based clearance in vascular circulation. By hitchhiking in situ onto specific blood components post-injection, NPs can selectively target tissue sites for unprecedentedly high drug delivery rates. Choline carboxylate ionic liquids (ILs) are biocompatible liquid salts <100X composed of bulky asymmetric cations and anions. This class of ILs has been previously shown to significantly extend circulation time and redirect biodistribution in BALB/c mice post-IV injection via hitchhiking on red blood cell (RBC) membranes. Herein, we synthesized & screened 60 choline carboxylic acid-based ILs to coat PLGA NPs and present the impact of structurally engineering the coordinated anion identity to selectively interface and hitchhike lymphocytes, monocytes, granulocytes, platelets, and RBCs in whole mouse blood for in situ targeted drug delivery. Furthermore, we find this nanoparticle platform to be biocompatible (non-cytotoxic), translate to human whole blood by resisting serum uptake and maintaining modest hitchhiking, and also significantly extend circulation retention over 24 hours in BALB/c healthy adult mice after IV injection. Because of their altered circulation profiles, we additionally observe dramatically different organ accumulation profiles compared to bare PLGA NPs. This study establishes an initial breakthrough platform for a modular and transformative targeting technology to hitchhike onto blood components with high efficacy and safety in the bloodstream post-IV administration.
Conflict of interest statement
COI Statement: EELT and CMH are named as inventors on intellectual property disclosures describing this work
Figures
Similar articles
-
Development of ionic liquid-coated PLGA nanoparticles for applications in intravenous drug delivery.Nat Protoc. 2023 Aug;18(8):2509-2557. doi: 10.1038/s41596-023-00843-6. Epub 2023 Jul 19. Nat Protoc. 2023. PMID: 37468651 Review.
-
Protein-avoidant ionic liquid (PAIL)-coated nanoparticles to increase bloodstream circulation and drive biodistribution.Sci Adv. 2020 Nov 25;6(48):eabd7563. doi: 10.1126/sciadv.abd7563. Print 2020 Nov. Sci Adv. 2020. PMID: 33239302 Free PMC article.
-
Ionic Liquid Coating-Driven Nanoparticle Delivery to the Brain: Applications for NeuroHIV.Adv Sci (Weinh). 2024 Jun;11(23):e2305484. doi: 10.1002/advs.202305484. Epub 2024 Apr 4. Adv Sci (Weinh). 2024. PMID: 38572510 Free PMC article.
-
Improved nanoformulation and bio-functionalization of linear-dendritic block copolymers with biocompatible ionic liquids.Nanoscale. 2022 Apr 21;14(16):6021-6036. doi: 10.1039/d2nr00538g. Nanoscale. 2022. PMID: 35362493
-
Applications of choline-based ionic liquids in drug delivery.Int J Pharm. 2022 Jan 25;612:121366. doi: 10.1016/j.ijpharm.2021.121366. Epub 2021 Dec 9. Int J Pharm. 2022. PMID: 34896216 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

