This is a preprint.
Estimating error rates for single molecule protein sequencing experiments
- PMID: 37502879
- PMCID: PMC10370102
- DOI: 10.1101/2023.07.18.549591
Estimating error rates for single molecule protein sequencing experiments
Update in
-
Estimating error rates for single molecule protein sequencing experiments.PLoS Comput Biol. 2024 Jul 5;20(7):e1012258. doi: 10.1371/journal.pcbi.1012258. eCollection 2024 Jul. PLoS Comput Biol. 2024. PMID: 38968291 Free PMC article.
Abstract
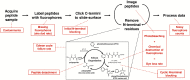
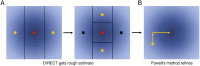
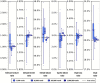
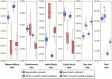
The practical application of new single molecule protein sequencing (SMPS) technologies requires accurate estimates of their associated sequencing error rates. Here, we describe the development and application of two distinct parameter estimation methods for analyzing SMPS reads produced by fluorosequencing. A Hidden Markov Model (HMM) based approach, extends whatprot, where we previously used HMMs for SMPS peptide-read matching. This extension offers a principled approach for estimating key parameters for fluorosequencing experiments, including missed amino acid cleavages, dye loss, and peptide detachment. Specifically, we adapted the Baum-Welch algorithm, a standard technique to estimate transition probabilities for an HMM using expectation maximization, but modified here to estimate a small number of parameter values directly rather than estimating every transition probability independently, which should help prevent overfitting. We demonstrate a high degree of accuracy on simulated data, but on experimental datasets, we observed that the model needed to be augmented with an additional error type, N-terminal blocking. This, in combination with data pre-processing, results in reasonable parameterizations of experimental datasets that agree with controlled experimental perturbations. A second independent implementation using a hybrid of DIRECT and Powell's method to reduce the root mean squared error (RMSE) between simulations and the real dataset was also developed. We compare these methods on both simulated and real data, finding that our Baum-Welch based approach outperforms DIRECT and Powell's method by most, but not all, criteria. Although some discrepancies between the results exist, we also find that both approaches provide similar error rate estimates from experimental single molecule fluorosequencing datasets.
Figures









References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
