This is a preprint.
GAGA zinc finger transcription factor searches chromatin by 1D-3D facilitated diffusion
- PMID: 37502885
- PMCID: PMC10369947
- DOI: 10.1101/2023.07.14.549009
GAGA zinc finger transcription factor searches chromatin by 1D-3D facilitated diffusion
Update in
-
GAGA zinc finger transcription factor searches chromatin by 1D-3D facilitated diffusion.Nat Struct Mol Biol. 2025 Aug 5. doi: 10.1038/s41594-025-01643-0. Online ahead of print. Nat Struct Mol Biol. 2025. PMID: 40764461
Abstract
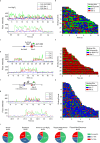
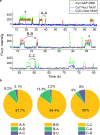
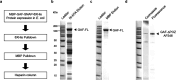
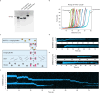
To elucidate how eukaryotic sequence-specific transcription factors (TFs) search for gene targets on chromatin, we used multi-color smFRET and single-particle imaging to track the diffusion of purified GAGA-Associated Factor (GAF) on DNA and nucleosomes. Monomeric GAF DNA-binding domain (DBD) bearing one zinc finger finds its cognate site by 1D or 3D diffusion on bare DNA and rapidly slides back-and-forth between naturally clustered motifs for seconds before escape. Multimeric, full-length GAF also finds clustered motifs on DNA by 1D-3D diffusion, but remains locked on target for longer periods. Nucleosome architecture effectively blocks GAF-DBD 1D-sliding into the histone core but favors retention of GAF-DBD when targeting solvent-exposed sites by 3D-diffusion. Despite the occlusive power of nucleosomes, 1D-3D facilitated diffusion enables GAF to effectively search for clustered cognate motifs in chromatin, providing a mechanism for navigation to nucleosome and nucleosome-free sites by a member of the largest TF family.
Figures




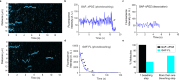
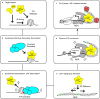





References
-
- Flury V. & Groth A. Safeguarding the epigenome through the cell cycle: a multitasking game. Curr. Opin. Genet. Dev. 85, 102161 (2024). - PubMed
-
- Li B., Carey M. & Workman J. L. The role of chromatin during transcription. Cell 128, 707–719 (2007). - PubMed
-
- Kadonaga J. T. Eukaryotic transcription: an interlaced network of transcription factors and chromatin-modifying machines. Cell 92, 307–313 (1998). - PubMed
-
- Isbel L., Grand R. S. & Schübeler D. Generating specificity in genome regulation through transcription factor sensitivity to chromatin. Nat. Rev. Genet. 23, 728–740 (2022). - PubMed
-
- Lukas J., Lukas C. & Bartek J. More than just a focus: The chromatin response to DNA damage and its role in genome integrity maintenance. Nat. Cell Biol. 13, 1161–1169 (2011). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
