This is a preprint.
Inhibiting tau-induced elevated nSMase2 activity and ceramides is therapeutic in murine Alzheimer's disease
- PMID: 37502930
- PMCID: PMC10371082
- DOI: 10.21203/rs.3.rs-3131295/v1
Inhibiting tau-induced elevated nSMase2 activity and ceramides is therapeutic in murine Alzheimer's disease
Update in
-
Inhibiting tau-induced elevated nSMase2 activity and ceramides is therapeutic in an Alzheimer's disease mouse model.Transl Neurodegener. 2023 Dec 4;12(1):56. doi: 10.1186/s40035-023-00383-9. Transl Neurodegener. 2023. PMID: 38049923 Free PMC article.
Abstract
Background: Cognitive decline in Alzheimer's disease (AD) is associated with prion-like tau propagation between neurons along synaptically connected networks, in part via extracellular vesicles (EV). EV biogenesis is triggered by ceramide enrichment at the plasma membrane from neutral sphingomyelinase2(nSMase2)-mediated cleavage of sphingomyelin. We report, for the first time, that tau expression triggers an elevation in brain ceramides and nSMase2 activity.
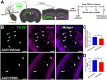
Methods: To determine the therapeutic benefit of inhibiting this elevation, we evaluated the efficacy of PDDC, the first potent, selective, orally bioavailable, and brain-penetrable nSMase2 inhibitor, in the PS19 tau transgenic AD murine model. Changes in brain ceramide and sphingomyelin levels, Tau content, histopathology, and nSMase2 target engagement were monitored, as well as changes in the number of brain-derived EVs in plasma and their Tau content. Additionally, we evaluated the ability of PDDC to impede tau propagation in a murine model where an adeno-associated virus(AAV) encoding for P301L/S320F double mutant human tau was stereotaxically-injected unilaterally into the hippocampus and the contralateral transfer to the dentate gyrus was monitored.
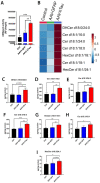
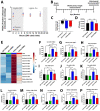
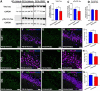
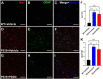
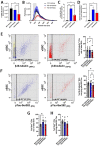
Results: Similar to human AD, PS19 mice exhibited increased brain ceramides and nSMase2 activity; both were completely normalized by PDDC treatment. PS19 mice exhibited elevated tau immunostaining, thinning of hippocampal neuronal cell layers, increased mossy fiber synaptophysin immunostaining, and glial activation, all pathologic features of human AD. PDDC treatment significantly attenuated these aberrant changes. Mouse plasma isolated from PDDC-treated PS19 mice exhibited reduced levels of neuron- and microglia-derived EVs, the former carrying lower phosphorylated Tau(pTau) levels, compared to untreated mice. In the AAV tau propagation model, PDDC normalized the tau-induced increase in brain ceramides and significantly decreased tau spreading to the contralateral side.
Conclusions: PDDC is a first-in-class therapeutic candidate that normalizes elevated brain ceramides and nSMase2 activity leading to the slowing of tau spread in AD mice.
Keywords: Alzheimer’s disease; ceramide; extracellular vesicles; neutral sphingomyelinase 2; tau.
Conflict of interest statement
Competing interests: C.T., A.G.T., R.R., and N.J.H and B.S.S. are listed as inventors in patent applications filed by Johns Hopkins Technology Ventures covering novel compositions and utilities of nSMase2 inhibitors, including PDDC. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict-of-interest policies. Other authors declare that no conflict of interest exist.
Figures

References
-
- Biogen, Biogen and Eisai Announce Design of ADUHELM ICARE AD-US Study, the First Real-World Observational Phase 4 Study in Alzheimer’s Disease at AAIC 2021. (2021).
-
- Braak H., Braak E., Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica 82, 239–259 (1991). - PubMed
-
- Ossenkoppele R., Pichet Binette A., Groot C., Smith R., Strandberg O., Palmqvist S., Stomrud E., Tideman P., Ohlsson T., Jogi J., Johnson K., Sperling R., Dore V., Masters C. L., Rowe C., Visser D., van Berckel B. N. M., van der Flier W. M., Baker S., Jagust W. J., Wiste H. J., Petersen R. C., Jack C. R. Jr., Hansson O., Amyloid and tau PET-positive cognitively unimpaired individuals are at high risk for future cognitive decline. Nature medicine 28, 2381–2387 (2022). - PMC - PubMed
-
- Simon D., Garcia-Garcia E., Royo F., Falcon-Perez J. M., Avila J., Proteostasis of tau. Tau overexpression results in its secretion via membrane vesicles. FEBS Lett 586, 47–54 (2012). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

