This is a preprint.
Sustaining wakefulness: Brainstem connectivity in human consciousness
- PMID: 37502983
- PMCID: PMC10369992
- DOI: 10.1101/2023.07.13.548265
Sustaining wakefulness: Brainstem connectivity in human consciousness
Abstract
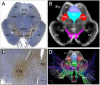
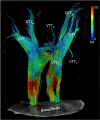
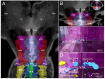
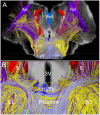
Consciousness is comprised of arousal (i.e., wakefulness) and awareness. Substantial progress has been made in mapping the cortical networks that modulate awareness in the human brain, but knowledge about the subcortical networks that sustain arousal is lacking. We integrated data from ex vivo diffusion MRI, immunohistochemistry, and in vivo 7 Tesla functional MRI to map the connectivity of a subcortical arousal network that we postulate sustains wakefulness in the resting, conscious human brain, analogous to the cortical default mode network (DMN) that is believed to sustain self-awareness. We identified nodes of the proposed default ascending arousal network (dAAN) in the brainstem, hypothalamus, thalamus, and basal forebrain by correlating ex vivo diffusion MRI with immunohistochemistry in three human brain specimens from neurologically normal individuals scanned at 600-750 μm resolution. We performed deterministic and probabilistic tractography analyses of the diffusion MRI data to map dAAN intra-network connections and dAAN-DMN internetwork connections. Using a newly developed network-based autopsy of the human brain that integrates ex vivo MRI and histopathology, we identified projection, association, and commissural pathways linking dAAN nodes with one another and with cortical DMN nodes, providing a structural architecture for the integration of arousal and awareness in human consciousness. We release the ex vivo diffusion MRI data, corresponding immunohistochemistry data, network-based autopsy methods, and a new brainstem dAAN atlas to support efforts to map the connectivity of human consciousness.
Conflict of interest statement
Competing interests: BF has a financial interest in CorticoMetrics, a company whose medical pursuits focus on brain imaging and measurement technologies. BF’s interests were reviewed and are managed by Massachusetts General Hospital and Mass General Brigham HealthCare in accordance with their conflict-of-interest policies.
Figures




References
-
- Buckner R. L., DiNicola L. M., The brain’s default network: updated anatomy, physiology and evolving insights. Nat Rev Neurosci 20, 593–608 (2019). - PubMed
-
- Koch C., Massimini M., Boly M., Tononi G., Neural correlates of consciousness: progress and problems. Nat Rev Neurosci 17, 307–321 (2016). - PubMed
-
- Kinney H. C., Korein J., Panigrahy A., Dikkes P., Goode R., Neuropathological findings in the brain of Karen Ann Quinlan. The role of the thalamus in the persistent vegetative state. N Engl J Med 330, 1469–1475 (1994). - PubMed
Publication types
Associated data
Grants and funding
- R21 EB018907/EB/NIBIB NIH HHS/United States
- K23 NS094538/NS/NINDS NIH HHS/United States
- P50 HD105351/HD/NICHD NIH HHS/United States
- RF1 MH121885/MH/NIMH NIH HHS/United States
- R01 NS105820/NS/NINDS NIH HHS/United States
- R01 EB023281/EB/NIBIB NIH HHS/United States
- R21 AG082082/AG/NIA NIH HHS/United States
- R01 EB006758/EB/NIBIB NIH HHS/United States
- UM1 MH130981/MH/NIMH NIH HHS/United States
- U01 MH093765/MH/NIMH NIH HHS/United States
- DP2 HD101400/HD/NICHD NIH HHS/United States
- R01 NS070963/NS/NINDS NIH HHS/United States
- U01 NS086625/NS/NINDS NIH HHS/United States
- R21 DC015888/DC/NIDCD NIH HHS/United States
- R01 AG064027/AG/NIA NIH HHS/United States
- R01 AG016495/AG/NIA NIH HHS/United States
- S10 RR019307/RR/NCRR NIH HHS/United States
- R01 AG070988/AG/NIA NIH HHS/United States
- RF1 MH123195/MH/NIMH NIH HHS/United States
- P41 RR014075/RR/NCRR NIH HHS/United States
- R21 HD095338/HD/NICHD NIH HHS/United States
- R01 AG008122/AG/NIA NIH HHS/United States
- R21 NS109627/NS/NINDS NIH HHS/United States
- R01 EB019956/EB/NIBIB NIH HHS/United States
- P41 EB030006/EB/NIBIB NIH HHS/United States
- UH3 NS095554/NS/NINDS NIH HHS/United States
- U01 MH117023/MH/NIMH NIH HHS/United States
- R21 NS072652/NS/NINDS NIH HHS/United States
- R01 HD102616/HD/NICHD NIH HHS/United States
- S10 RR023043/RR/NCRR NIH HHS/United States
- R01 NS083534/NS/NINDS NIH HHS/United States
- S10 RR023401/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
