This is a preprint.
Native dynamics and allosteric responses in PTP1B probed by high-resolution HDX-MS
- PMID: 37503000
- PMCID: PMC10369962
- DOI: 10.1101/2023.07.12.548582
Native dynamics and allosteric responses in PTP1B probed by high-resolution HDX-MS
Update in
-
Native dynamics and allosteric responses in PTP1B probed by high-resolution HDX-MS.Protein Sci. 2024 Jun;33(6):e5024. doi: 10.1002/pro.5024. Protein Sci. 2024. PMID: 38801229 Free PMC article.
Abstract
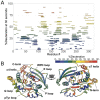
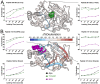
Protein tyrosine phosphatase 1B (PTP1B) is a validated therapeutic target for obesity, diabetes, and certain types of cancer. In particular, allosteric inhibitors hold potential for therapeutic use, but an incomplete understanding of conformational dynamics and allostery in this protein has hindered their development. Here, we interrogate solution dynamics and allosteric responses in PTP1B using high-resolution hydrogen-deuterium exchange mass spectrometry (HDX-MS), an emerging and powerful biophysical technique. Using HDX-MS, we obtain a detailed map of the solution dynamics of apo PTP1B, revealing several flexible loops interspersed among more constrained and rigid regions within the protein structure, as well as local regions that exchange faster than expected from their secondary structure and buriedness. We demonstrate that our HDX rate data obtained in solution adds value to predictions of dynamics derived from a pseudo-ensemble constructed from ~200 crystal structures of PTP1B. Furthermore, we report HDX-MS maps for PTP1B with active-site vs. allosteric small-molecule inhibitors. These maps reveal distinct, dramatic, and widespread effects on protein dynamics relative to the apo form, including changes to dynamics in locations distal (>35 Å) from the respective ligand binding sites. These results help shed light on the allosteric nature of PTP1B and the surprisingly far-reaching consequences of inhibitor binding in this important protein. Overall, our work showcases the potential of HDX-MS for elucidating protein conformational dynamics and allosteric effects of small-molecule ligands, and highlights the potential of integrating HDX-MS alongside other complementary methods to guide the development of new therapeutics.
Figures



References
-
- Qian S, Zhang M, He Y, Wang W, Liu S (2016) Recent advances in the development of protein tyrosine phosphatase 1B inhibitors for Type 2 diabetes. Future Med. Chem. 8:1239–1258. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
