This is a preprint.
Regression and Alignment for Functional Data and Network Topology
- PMID: 37503017
- PMCID: PMC10370026
- DOI: 10.1101/2023.07.13.548836
Regression and Alignment for Functional Data and Network Topology
Update in
-
Regression and alignment for functional data and network topology.Biostatistics. 2024 Dec 31;26(1):kxae026. doi: 10.1093/biostatistics/kxae026. Biostatistics. 2024. PMID: 39140988 Free PMC article.
Abstract
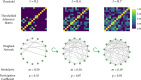
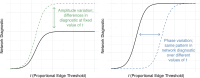
In the brain, functional connections form a network whose topological organization can be described by graph-theoretic network diagnostics. These include characterizations of the community structure, such as modularity and participation coefficient, which have been shown to change over the course of childhood and adolescence. To investigate if such changes in the functional network are associated with changes in cognitive performance during development, network studies often rely on an arbitrary choice of pre-processing parameters, in particular the proportional threshold of network edges. Because the choice of parameter can impact the value of the network diagnostic, and therefore downstream conclusions, we propose to circumvent that choice by conceptualizing the network diagnostic as a function of the parameter. As opposed to a single value, a network diagnostic curve describes the connectome topology at multiple scales-from the sparsest group of the strongest edges to the entire edge set. To relate these curves to executive function and other covariates, we use scalar-on-function regression, which is more flexible than previous functional data-based models used in network neuroscience. We then consider how systematic differences between networks can manifest in misalignment of diagnostic curves, and consequently propose a supervised curve alignment method that incorporates auxiliary information from other variables. Our algorithm performs both functional regression and alignment via an iterative, penalized, and nonlinear likelihood optimization. The illustrated method has the potential to improve the interpretability and generalizability of neuroscience studies where the goal is to study heterogeneity among a mixture of function- and scalar-valued measures.
Keywords: Alignment; Functional Data Analysis; Functional Regression; Network Neuroscience.
Conflict of interest statement
Conflict of Interest: None declared.
Figures



References
-
- Baum Graham L., Ciric Rastko, Roalf David R., Betzel Richard F., Moore Tyler M., Shinohara Russell T., Kahn Ari E., Vandekar Simon N., Rupert Petra E., Quarmley Megan, Cook Philip A., Elliott Mark A., Ruparel Kosha, Gur Raquel E., Gur Ruben C., Bassett Danielle S. and others. (2017, June). Modular segregation of structural brain networks supports the development of executive function in youth. Current Biology 27(11), 1561–1572.e8. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
