This is a preprint.
Complete Protection from SARS-CoV-2 Lung Infection in Mice Through Combined Intranasal Delivery of PIKfyve Kinase and TMPRSS2 Protease Inhibitors
- PMID: 37503261
- PMCID: PMC10370096
- DOI: 10.1101/2023.07.19.549731
Complete Protection from SARS-CoV-2 Lung Infection in Mice Through Combined Intranasal Delivery of PIKfyve Kinase and TMPRSS2 Protease Inhibitors
Abstract
Emerging variants of concern of SARS-CoV-2 can significantly reduce the prophylactic and therapeutic efficacy of vaccines and neutralizing antibodies due to mutations in the viral genome. Targeting cell host factors required for infection provides a complementary strategy to overcome this problem since the host genome is less susceptible to variation during the life span of infection. The enzymatic activities of the endosomal PIKfyve phosphoinositide kinase and the serine protease TMPRSS2 are essential to meditate infection in two complementary viral entry pathways. Simultaneous inhibition in cultured cells of their enzymatic activities with the small molecule inhibitors apilimod dimesylate and nafamostat mesylate synergistically prevent viral entry and infection of native SARS-CoV-2 and vesicular stomatitis virus (VSV)-SARS-CoV-2 chimeras expressing the SARS-CoV-2 surface spike (S) protein and of variants of concern. We now report prophylactic prevention of lung infection in mice intranasally infected with SARS-CoV-2 beta by combined intranasal delivery of very low doses of apilimod dimesylate and nafamostat mesylate, in a formulation that is stable for over 3 months at room temperature. Administration of these drugs up to 6 hours post infection did not inhibit infection of the lungs but substantially reduced death of infected airway epithelial cells. The efficiency and simplicity of formulation of the drug combination suggests its suitability as prophylactic or therapeutic treatment against SARS-CoV-2 infection in households, point of care facilities, and under conditions where refrigeration would not be readily available.
Figures
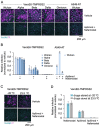
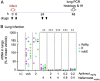



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
