This is a preprint.
Ebola Virus VP35 Interacts Non-Covalently with Ubiquitin Chains to Promote Viral Replication Creating New Therapeutic Opportunities
- PMID: 37503276
- PMCID: PMC10369991
- DOI: 10.1101/2023.07.14.549057
Ebola Virus VP35 Interacts Non-Covalently with Ubiquitin Chains to Promote Viral Replication Creating New Therapeutic Opportunities
Update in
-
Ebola virus VP35 interacts non-covalently with ubiquitin chains to promote viral replication.PLoS Biol. 2024 Feb 29;22(2):e3002544. doi: 10.1371/journal.pbio.3002544. eCollection 2024 Feb. PLoS Biol. 2024. PMID: 38422166 Free PMC article.
Abstract
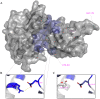
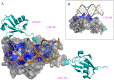
Ebolavirus (EBOV) belongs to a family of highly pathogenic viruses that cause severe hemorrhagic fever in humans. EBOV replication requires the activity of the viral polymerase complex, which includes the co-factor and Interferon antagonist VP35. We previously showed that the covalent ubiquitination of VP35 promotes virus replication by regulating interactions with the polymerase complex. In addition, VP35 can also interact non-covalently with ubiquitin (Ub); however, the function of this interaction is unknown. Here, we report that VP35 interacts with free (unanchored) K63-linked polyUb chains. Ectopic expression of Isopeptidase T (USP5), which is known to degrade unanchored polyUb chains, reduced VP35 association with Ub and correlated with diminished polymerase activity in a minigenome assay. Using computational methods, we modeled the VP35-Ub non-covalent interacting complex, identified the VP35-Ub interacting surface and tested mutations to validate the interface. Docking simulations identified chemical compounds that can block VP35-Ub interactions leading to reduced viral polymerase activity that correlated with reduced replication of infectious EBOV. In conclusion, we identified a novel role of unanchored polyUb in regulating Ebola virus polymerase function and discovered compounds that have promising anti-Ebola virus activity.
Keywords: Biological Science; Computational biology; Ebola virus; Microbiology and Biophysics and Computational Biology; Polyubiquitin; VP35; antivirals; viral polymerase.
Conflict of interest statement
Competing Interest Statement: The authors declare no competing interest.
Figures




Similar articles
-
Ebola virus VP35 interacts non-covalently with ubiquitin chains to promote viral replication.PLoS Biol. 2024 Feb 29;22(2):e3002544. doi: 10.1371/journal.pbio.3002544. eCollection 2024 Feb. PLoS Biol. 2024. PMID: 38422166 Free PMC article.
-
The Host E3-Ubiquitin Ligase TRIM6 Ubiquitinates the Ebola Virus VP35 Protein and Promotes Virus Replication.J Virol. 2017 Aug 24;91(18):e00833-17. doi: 10.1128/JVI.00833-17. Print 2017 Sep 15. J Virol. 2017. PMID: 28679761 Free PMC article.
-
Cynarin blocks Ebola virus replication by counteracting VP35 inhibition of interferon-beta production.Antiviral Res. 2022 Feb;198:105251. doi: 10.1016/j.antiviral.2022.105251. Epub 2022 Jan 20. Antiviral Res. 2022. PMID: 35066016
-
Ubiquitination of Ebola virus VP35 at lysine 309 regulates viral transcription and assembly.PLoS Pathog. 2022 May 9;18(5):e1010532. doi: 10.1371/journal.ppat.1010532. eCollection 2022 May. PLoS Pathog. 2022. PMID: 35533195 Free PMC article.
-
Insights into Ebola Virus VP35 and VP24 Interferon Inhibitory Functions and their Initial Exploitation as Drug Targets.Infect Disord Drug Targets. 2019;19(4):362-374. doi: 10.2174/1871526519666181123145540. Infect Disord Drug Targets. 2019. PMID: 30468131 Review.
References
-
- Baseler L., Chertow D. S., Johnson K. M., Feldmann H., Morens D. M., The Pathogenesis of Ebola Virus Disease*. Annual Review of Pathology: Mechanisms of Disease 12, 387–418 (2017). - PubMed
-
- Malvy D., McElroy A. K., de Clerck H., Günther S., van Griensven J., Ebola virus disease. The Lancet 393, 936–948 (2019). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
