Development of conjugated secondary antibodies for wildlife disease surveillance
- PMID: 37503338
- PMCID: PMC10368751
- DOI: 10.3389/fimmu.2023.1221071
Development of conjugated secondary antibodies for wildlife disease surveillance
Abstract
Disease monitoring in free-ranging wildlife is a challenge and often relies on passive surveillance. Alternatively, proactive surveillance that relies on the detection of specific antibodies could give more reliable and timely insight into disease presence and prevalence in a population, especially if the evidence of disease occurs below detection thresholds for passive surveillance. Primary binding assays, like the indirect ELISA for antibody detection in wildlife, are hampered by a lack of species-specific conjugates. In this study, we developed anti-kudu (Tragelaphus strepsiceros) and anti-impala (Aepyceros melampus) immunoglobulin-specific conjugates in chickens and compared them to the binding of commercially available protein-G and protein-AG conjugates, using an ELISA-based avidity index. The conjugates were evaluated for cross-reaction with sera from other wild herbivores to assess future use in ELISAs. The developed conjugates had a high avidity of >70% against kudu and impala sera. The commercial conjugates (protein-G and protein-AG) had significantly low relative avidity (<20%) against these species. Eighteen other wildlife species demonstrated cross-reactivity with a mean relative avidity of >50% with the impala and kudu conjugates and <40% with the commercial conjugates. These results demonstrate that species-specific conjugates are important tools for the development and validation of immunoassays in wildlife and for the surveillance of zoonotic agents along the livestock-wildlife-human interface.
Keywords: adaptive immunity; avidity; conjugates; diagnostics; enzyme-linked immunosorbent assay (ELISA); passive disease surveillance; wildlife species.
Copyright © 2023 Ochai, Crafford, Kamath, Turner and van Heerden.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
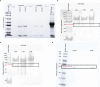
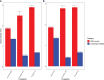
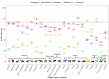

References
-
- Artois M, Bengis R, Delahay RJ, Duchêne M-J, Duff JP, Ferroglio E, et al. . Wildlife disease surveillance and monitoring. Springer Japan; (2009) p:187–213. doi: 10.1007/978-4-431-77134-0_10 - DOI
-
- Garnier R, Ramos R, Sanz-Aguilar A, Poisbleau M, Weimerskirch H, Burthe S, et al. . Interpreting ELISA analyses from wild animal samples: some recurrent issues and solutions. Funct Ecol (2017) 31(12):2255–62. doi: 10.1111/1365-2435.12942 - DOI
-
- Kock ND, Jongejan F, Kock MD, Kock RA, Morkel P. Serological evidence for cowdria ruminantium infection in free-ranging black (Diceros bicornis) and white (Ceratotherium simum) rhinoceroses in Zimbabwe. J Zoo Wildlife Med (1992) 23(4):409–13.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

