NAD+, Axonal Maintenance, and Neurological Disease
- PMID: 37503611
- PMCID: PMC10715442
- DOI: 10.1089/ars.2023.0350
NAD+, Axonal Maintenance, and Neurological Disease
Abstract
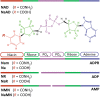
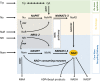
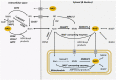
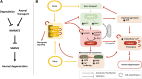
Significance: The remarkable geometry of the axon exposes it to unique challenges for survival and maintenance. Axonal degeneration is a feature of peripheral neuropathies, glaucoma, and traumatic brain injury, and an early event in neurodegenerative diseases. Since the discovery of Wallerian degeneration (WD), a molecular program that hijacks nicotinamide adenine dinucleotide (NAD+) metabolism for axonal self-destruction, the complex roles of NAD+ in axonal viability and disease have become research priority. Recent Advances: The discoveries of the protective Wallerian degeneration slow (WldS) and of sterile alpha and TIR motif containing 1 (SARM1) activation as the main instructive signal for WD have shed new light on the regulatory role of NAD+ in axonal degeneration in a growing number of neurological diseases. SARM1 has been characterized as a NAD+ hydrolase and sensor of NAD+ metabolism. The discovery of regulators of nicotinamide mononucleotide adenylyltransferase 2 (NMNAT2) proteostasis in axons, the allosteric regulation of SARM1 by NAD+ and NMN, and the existence of clinically relevant windows of action of these signals has opened new opportunities for therapeutic interventions, including SARM1 inhibitors and modulators of NAD+ metabolism. Critical Issues: Events upstream and downstream of SARM1 remain unclear. Furthermore, manipulating NAD+ metabolism, an overdetermined process crucial in cell survival, for preventing the degeneration of the injured axon may be difficult and potentially toxic. Future Directions: There is a need for clarification of the distinct roles of NAD+ metabolism in axonal maintenance as contrasted to WD. There is also a need to better understand the role of NAD+ metabolism in axonal endangerment in neuropathies, diseases of the white matter, and the early stages of neurodegenerative diseases of the central nervous system. Antioxid. Redox Signal. 39, 1167-1184.
Keywords: SARM1; Wallerian degeneration; axonal degeneration; neurodegeneration; nicotinamide; pellagra.
Conflict of interest statement
No competing financial interests exist.
Figures




Similar articles
-
Protective effects of NAMPT or MAPK inhibitors and NaR on Wallerian degeneration of mammalian axons.Neurobiol Dis. 2022 Sep;171:105808. doi: 10.1016/j.nbd.2022.105808. Epub 2022 Jun 30. Neurobiol Dis. 2022. PMID: 35779777 Free PMC article.
-
SARM1 activation triggers axon degeneration locally via NAD⁺ destruction.Science. 2015 Apr 24;348(6233):453-7. doi: 10.1126/science.1258366. Epub 2015 Apr 23. Science. 2015. PMID: 25908823 Free PMC article.
-
Molecular mechanisms in the initiation phase of Wallerian degeneration.Eur J Neurosci. 2016 Aug;44(4):2040-8. doi: 10.1111/ejn.13250. Epub 2016 May 30. Eur J Neurosci. 2016. PMID: 27062141 Review.
-
Mechanism of initiation and regulation of axonal degeneration with special reference to NMNATs and Sarm1.Neurosci Res. 2023 Dec;197:3-8. doi: 10.1016/j.neures.2021.11.002. Epub 2021 Nov 9. Neurosci Res. 2023. PMID: 34767875 Review.
-
Mitochondrial impairment activates the Wallerian pathway through depletion of NMNAT2 leading to SARM1-dependent axon degeneration.Neurobiol Dis. 2020 Feb;134:104678. doi: 10.1016/j.nbd.2019.104678. Epub 2019 Nov 15. Neurobiol Dis. 2020. PMID: 31740269 Free PMC article.
Cited by
-
SARM1: The Checkpoint of Axonal Degeneration in the Nervous System Disorders.Mol Neurobiol. 2025 Jul;62(7):9240-9257. doi: 10.1007/s12035-025-04835-3. Epub 2025 Mar 17. Mol Neurobiol. 2025. PMID: 40097763 Review.
-
NAD+ Precursors Reverse Experimental Diabetic Neuropathy in Mice.Int J Mol Sci. 2024 Jan 16;25(2):1102. doi: 10.3390/ijms25021102. Int J Mol Sci. 2024. PMID: 38256175 Free PMC article.
-
Genetic ablation of Sarm1 attenuates expression and mislocalization of phosphorylated TDP-43 after mouse repetitive traumatic brain injury.Acta Neuropathol Commun. 2023 Dec 20;11(1):206. doi: 10.1186/s40478-023-01709-4. Acta Neuropathol Commun. 2023. PMID: 38124145 Free PMC article.
-
SARM1 in the pathogenesis of immune-related disease.Toxicol Res (Camb). 2024 Dec 8;13(6):tfae208. doi: 10.1093/toxres/tfae208. eCollection 2024 Dec. Toxicol Res (Camb). 2024. PMID: 39664502 Review.
-
Human Conjunctival Transcriptome in Acanthamoeba Keratitis: An Exploratory Study.Cornea. 2024 Oct 1;43(10):1272-1277. doi: 10.1097/ICO.0000000000003545. Epub 2024 May 21. Cornea. 2024. PMID: 38771726
References
-
- Adalbert R, Coleman MP. Review: Axon pathology in age-related neurodegenerative disorders. Neuropathol Appl Neurobiol 2013;39, 90–108. - PubMed
-
- Adalbert R, Nogradi A, Szabo A, et al. . The slow Wallerian degeneration gene in vivo protects motor axons but not their cell bodies after avulsion and neonatal axotomy. Eur J Neurosci 2006;24:2163–2168. - PubMed
-
- Aksoy P, White TA, Thompson M, et al. . Regulation of intracellular levels of NAD: A novel role for CD38. Biochem Biophys Res Commun 2006;345:1386–1392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
