Low‑dose ionizing radiation attenuates high glucose‑induced hepatic apoptosis and immune factor release via modulation of a miR‑155‑SOCS1 axis
- PMID: 37503757
- PMCID: PMC10433713
- DOI: 10.3892/mmr.2023.13058
Low‑dose ionizing radiation attenuates high glucose‑induced hepatic apoptosis and immune factor release via modulation of a miR‑155‑SOCS1 axis
Abstract
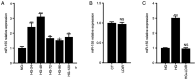
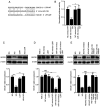
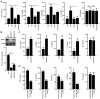
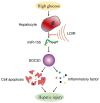
Diabetic liver injury (DLI) can result in several diseases of the liver, including steatohepatitis, liver fibrosis, cirrhosis, and liver cancer. Low‑dose ionizing radiation (LDIR) has hormetic effects in normal/disease conditions. However, whether LDIR has a beneficial effect on DLI has not been assessed previously. MicroRNA (miR)‑155 and its target gene suppressor of cytokine signaling 1 (SOCS1) play critical roles in modulating hepatic proliferation, apoptosis, and immunity. However, whether a miR‑155‑SOCS1 axis is involved in high glucose (HG) induced hepatic damage remains to be determined. In the present study, mouse hepatocyte AML12 cells were treated with 30 mM glucose (HG), 75 mGy X‑ray (LDIR), or HG plus LDIR. The expression levels of miR‑155 and SOCS1 were determined by reverse transcription‑quantitative PCR and western blotting. Additionally, apoptosis was measured using flow cytometry. The release of inflammatory factors, including TNF‑α, IL‑1β, IL‑6, IL‑10, and IFN‑γ, after HG and/or LDIR treatment was detected by ELISA. The results showed that HG may induce hepatic apoptosis by upregulating the levels of miR‑155 and downregulating the levels of SOCS1. HG also stimulated the secretion of TNF‑α, IL‑1β, IL‑6, and IL‑10. However, LDIR blocked the HG‑induced activation of a miR‑155‑SOCS1 axis and suppressed the release of inflammatory factors. These results indicated that a miR‑155‑SOCS1 axis plays a role in HG‑induced liver injury, and LDIR may exert a hepatoprotective effect by regulating the miR‑155‑SOCS1 axis.
Keywords: hepatic injury; high glucose; low‑dose ionizing radiation; microRNA‑155; suppressor of cytokine signaling 1.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Inhibition of microRNA‑155 relieves sepsis‑induced liver injury through inactivating the JAK/STAT pathway.Mol Med Rep. 2015 Oct;12(4):6013-8. doi: 10.3892/mmr.2015.4188. Epub 2015 Aug 5. Mol Med Rep. 2015. PMID: 26251957
-
Piperazine ferulate attenuates high glucose‑induced mesangial cell injury via the regulation of p66Shc.Mol Med Rep. 2021 May;23(5):374. doi: 10.3892/mmr.2021.12013. Epub 2021 Mar 24. Mol Med Rep. 2021. PMID: 33760157 Free PMC article.
-
MicroRNA-378 promotes hepatic inflammation and fibrosis via modulation of the NF-κB-TNFα pathway.J Hepatol. 2019 Jan;70(1):87-96. doi: 10.1016/j.jhep.2018.08.026. Epub 2018 Sep 13. J Hepatol. 2019. PMID: 30218679 Free PMC article.
-
Therapeutic potential of low dose ionizing radiation against cancer, dementia, and diabetes: evidences from epidemiological, clinical, and preclinical studies.Mol Biol Rep. 2023 Mar;50(3):2823-2834. doi: 10.1007/s11033-022-08211-5. Epub 2023 Jan 3. Mol Biol Rep. 2023. PMID: 36595119 Free PMC article. Review.
-
Molecular mechanisms of low dose ionizing radiation-induced hormesis, adaptive responses, radioresistance, bystander effects, and genomic instability.Int J Radiat Biol. 2015 Jan;91(1):13-27. doi: 10.3109/09553002.2014.937510. Epub 2014 Aug 21. Int J Radiat Biol. 2015. PMID: 24975555 Review.
Cited by
-
Low-Dose Ionizing Radiation and Male Reproductive Immunity: Elucidating Subtle Modulations and Long-Term Health Implications.Int J Mol Sci. 2025 Mar 4;26(5):2269. doi: 10.3390/ijms26052269. Int J Mol Sci. 2025. PMID: 40076897 Free PMC article. Review.
References
-
- Mettler FA, Sinclair WK, Anspaugh L, Edington C, Harley JH, Ricks RC, Selby PB, Webster EW, Wyckoff HO. The 1986 and 1988 UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation) reports: Findings and implications. Health Phys. 1990;58:241–250. doi: 10.1097/00004032-199003000-00001. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

