A pilot in vivo study: potential ovarian cancer therapeutic by placental extracellular vesicles
- PMID: 37503762
- PMCID: PMC10442519
- DOI: 10.1042/BSR20230307
A pilot in vivo study: potential ovarian cancer therapeutic by placental extracellular vesicles
Abstract
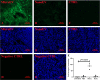
The biological links between cancer and pregnancy are of interest due to parallel proliferative, immunosuppressive, and invasive mechanisms between tumour and placental cells. However, the proliferation and invasion of placental cells are strictly regulated. The understanding of this regulation is largely unknown. Placental extracellular vesicles (EVs) may play an important role in this regulation, as placental EVs are known to contribute to maternal adaptation, including adaptation of the vascular and immune systems. We have previously reported that placental EVs significantly inhibited ovarian cancer cell proliferation by delaying the progression of the cell cycle. We, therefore, performed this pilot in vivo study to investigate whether placental EVs can also inhibit ovarian tumour growth in a SKOV-3 human tumour xenograft model. A single intraperitoneal injection of placental EVs at 15 days post tumour implantation, significantly inhibited the growth of the tumours in our in vivo model. Signs of cellular necrosis were observed in the ovarian tumour tissues, but not in other organs collected from mice that had been treated with placental EVs. Expression of receptor-interacting kinase 1 (RIPK1) and mixed linkage kinase domain-like (MLKL), which are mediators of necroptosis were not observed in our xenografted tumours. However, extensive infiltration of CD169+ macrophages and NK cells in ovarian tumour tissues collected from placental micro-EVs treated mice were observed. We demonstrate here that inhibition of ovarian tumour growth in our xenograft model by placental EVs involves cellular necrosis and infiltration of CD169+ macrophages and NK cells into the tumour tissues.
Keywords: CD169+; necrosis; ovarian cancer; placental EVs; tumour regression; tumour xenograft model.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
This
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

