Clinical covariates that improve the description of high dose methotrexate pharmacokinetics in a diverse population to inform MTXPK.org
- PMID: 37503924
- PMCID: PMC10651646
- DOI: 10.1111/cts.13600
Clinical covariates that improve the description of high dose methotrexate pharmacokinetics in a diverse population to inform MTXPK.org
Abstract
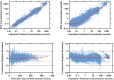
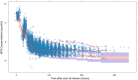
The MTXPK.org webtool was launched in December 2019 and was developed to facilitate model-informed supportive care and optimal use of glucarpidase following the administration of high-dose methotrexate (HDMTX). One limitation identified during the original development of the MTXPK.org tool was the perceived generalizability because the modeled population comprised solely of Nordic pediatric patients receiving 24-h infusions for the treatment of acute lymphoblastic leukemia. The goal of our study is to describe the pharmacokinetics of HDMTX from a diverse patient population (e.g., races, ethnicity, indications for methotrexate, and variable infusion durations) and identify meaningful factors that account for methotrexate variability and improve the model's performance. To do this, retrospectively analyzed pharmacokinetic and toxicity data from pediatric and adolescent young adult patients who were receiving HDMTX (>0.5 g/m2 ) for the treatment of a cancer diagnosis from three pediatric medical centers. We performed population pharmacokinetic modeling referencing the original MTXPK.org NONMEM model (includes body surface area and serum creatinine as covariates) on 1668 patients, 7506 administrations of HDMTX, and 30,250 concentrations. Our results support the parameterizations of short infusion duration (<8 h) and the presence of Down syndrome on methotrexate clearance, the parameterization of severe hypoalbuminemia (<2.5 g/dL) on the intercompartmental clearance (Q2 and Q3), and the parameterization of pleural effusion on the volume of distribution (V1 and V2). These novel parameterizations will increase the generalizability of the MTXPK.org model once they are added to the webtool.
© 2023 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
L.B.R. consulted for and received research funding from BTG, International. M.M.O. received honoraria from Pfizer, consulted for Jazz Pharmaceuticals, and research funding from AbbVie, Amgen, and Pfizer. M.B.B. has received compensation as a member of a scientific advisory board for BTG, International, research funding from Bristol Myers Squibb, Celgene, and consulted for Jazz Pharmaceuticals. T.P.M. has stock and other ownership interests in AbbVie, Gilead Sciences, Thermo Fisher Scientific, and United Health Group. All other authors declared no competing interests for this work.
Figures
References
-
- BTG International I . Voraxaze (glucarpidase) package insert. 2012.
-
- Health UDo, Services H . Common terminology criteria for adverse events. Version 5.0. Published November 27, 2017 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

