Magnetically Assisted Immobilization-Free Detection of microRNAs Based on the Signal Amplification of Duplex-Specific Nuclease
- PMID: 37504098
- PMCID: PMC10437004
- DOI: 10.3390/bios13070699
Magnetically Assisted Immobilization-Free Detection of microRNAs Based on the Signal Amplification of Duplex-Specific Nuclease
Abstract
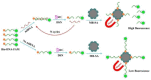
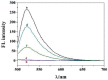
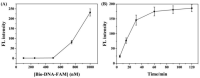
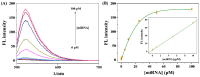
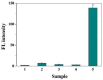
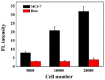
The double specific nuclease (DSN)-based methods for microRNAs (miRNAs) detection usually require the immobilization of DNA probes on a solid surface. However, such strategies have the drawbacks of low hybridization and cleavage efficiency caused by steric hindrance effect and high salt concentration on the solid surface. Herein, we proposed an immobilization-free method for miRNA detection on the basic of DSN-assisted signal amplification. The biotin- and fluorophore-labeled probes were captured by streptavidin-modified magnetic beads through streptavidin-biotin interactions, thus producing a poor fluorescence signal. Once the DNA probes were hybridized with target miRNA in solution to form DNA-miRNA duplexes, DNA stands in the duplexes would be selectively digested by DSN. The released target miRNA could initiate the next hybridization/cleavage recycling in the homogeneous solution, finally resulting in the release of numerous fluorophore-labeled fragments. The released fluorophores remained in solution and emitted strong fluorescence after treatment by the streptavidin-modified magnetic beads. The immobilization-free method achieved the assays of miRNA-21 with a detection limit down to 0.01 pM. It was employed to evaluate the expression levels of miRNA-21 in different cancer cells with satisfactory results.
Keywords: double specific nuclease; homogeneous analysis; immobilization-free; magnetic bead; microRNAs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Duplex-specific nuclease-based electrochemical biosensor for the detection of microRNAs by conversion of homogeneous assay into surface-tethered electrochemical analysis.Anal Chim Acta. 2021 Mar 8;1149:338199. doi: 10.1016/j.aca.2021.338199. Epub 2021 Jan 7. Anal Chim Acta. 2021. PMID: 33551055
-
Colorimetric and fluorescent dual-mode detection of microRNA based on duplex-specific nuclease assisted gold nanoparticle amplification.Analyst. 2019 Aug 21;144(16):4917-4924. doi: 10.1039/c9an01013k. Epub 2019 Jul 17. Analyst. 2019. PMID: 31313769
-
A simple and highly sensitive fluorescence assay for microRNAs.Analyst. 2015 Mar 21;140(6):1932-8. doi: 10.1039/c4an02146k. Epub 2015 Feb 6. Analyst. 2015. PMID: 25655238
-
State-of-the-Art Fluorescent Probes: Duplex-Specific Nuclease-Based Strategies for Early Disease Diagnostics.Biosensors (Basel). 2022 Dec 15;12(12):1172. doi: 10.3390/bios12121172. Biosensors (Basel). 2022. PMID: 36551139 Free PMC article. Review.
-
Recent advances in duplex-specific nuclease-based signal amplification strategies for microRNA detection.Biosens Bioelectron. 2020 Oct 1;165:112449. doi: 10.1016/j.bios.2020.112449. Epub 2020 Jul 14. Biosens Bioelectron. 2020. PMID: 32745963 Review.
Cited by
-
An electrochemical aptasensor for detection of Helicobacter pylori based on AuNPs and AgNPs-GO nanoparticles.Front Bioeng Biotechnol. 2025 Jul 9;13:1619336. doi: 10.3389/fbioe.2025.1619336. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40704101 Free PMC article.
-
Efficient solid-phase extraction of oligo-DNA from complex media using a nitrocellulose membrane modified with carbon nanotubes and aminated reduced graphene oxide.Sci Rep. 2025 Feb 13;15(1):5325. doi: 10.1038/s41598-025-89705-7. Sci Rep. 2025. PMID: 39948136 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

