Expanding the Horizons of Pre-Transplant Renal Vascular Assessment Using Ex Vivo Perfusion
- PMID: 37504261
- PMCID: PMC10378498
- DOI: 10.3390/cimb45070345
Expanding the Horizons of Pre-Transplant Renal Vascular Assessment Using Ex Vivo Perfusion
Abstract
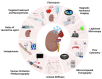
Recently, immense efforts have focused on improving the preservation of (sub)optimal donor organs by means of ex vivo perfusion, which enables the opportunity for organ reconditioning and viability assessment. However, there is still no biomarker that correlates with renal viability. Therefore, it is essential to explore new techniques for pre-transplant assessment of organ quality to guarantee successful long-term transplantation outcomes. The renal vascular compartment has received little attention in machine perfusion studies. In vivo, proper renal vascular and endothelial function is essential for maintaining homeostasis and long-term graft survival. In an ex vivo setting, little is known about vascular viability and its implications for an organ's suitability for transplant. Seeing that endothelial damage is the first step in a cascade of disruptions and maintaining homeostasis is crucial for positive post-transplant outcomes, further research is key to clarifying the (patho)physiology of the renal vasculature during machine perfusion. In this review, we aim to summarize key aspects of renal vascular physiology, describe the role of the renal vasculature in pathophysiological settings, and explain how ex vivo perfusion plays a role in either unveiling or targeting such processes. Additionally, we discuss potentially new vascular assessment tools during ex vivo renal perfusion.
Keywords: endothelial cells; ex vivo perfusion; homeostasis; kidney; transplantation; vasculature; viability assessment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Abad J.B., Tolosa Eizaguirre E., Mayans A.R., Rosell Costa D., Robles García J.E., Zudaire Bergera J., Berián Polo J.M., Pascual Piedrola I. Influence of Donor Age on Graft Survival. 2009. [(accessed on 2 April 2023)]. Available online: www.elsevier.es/actasuro. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

