Exosome Analysis in Prostate Cancer: How They Can Improve Biomarkers' Performance
- PMID: 37504300
- PMCID: PMC10378661
- DOI: 10.3390/cimb45070384
Exosome Analysis in Prostate Cancer: How They Can Improve Biomarkers' Performance
Abstract
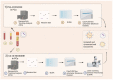
Exosomes are extracellular nanovesicles (EV), that is, carriers of different biomolecules such as lipids, proteins, nucleic acids. Their composition and the fact that their release dramatically increases in cases of tumorigenesis open up different scenarios on their possible application to research into new biomarkers. The first purpose of the present review was to specifically analyze and compare different methodologies available for the use of exosomes in prostate cancer (PC). The most widely applied methodologies include ultracentrifugation techniques, size-based techniques, immunoaffinity capture-based techniques (mainly ELISA), and precipitation. To optimize the acquisition of exosomes from the reference sample, more techniques can be applied in sequence for a single extraction, thereby determining an increase in labor time and costs. The second purpose was to describe clinical results obtained with the analysis of PSA-expressing exosomes in PC; this provides an incredibly accurate method of discriminating between healthy patients and those with prostate disease. Specifically, the IC-ELISA alone method achieved 98.57% sensitivity and 80.28% specificity in discriminating prostate cancer (PC) from benign prostatic hyperplasia (BPH). An immunocapture-based ELISA assay was performed to quantify and characterize carbonic anhydrase (CA) IX expression in exosomes. The results revealed that CA IX positive exosomes were 25-fold higher in plasma samples from PC patients than in those from healthy controls. The analysis of PC-linked exosomes represents a promising diagnostic model that can effectively distinguish patients with PC from those with non-malignant prostatic disease. However, the use of exosome analysis in clinical practice is currently limited by several issues, including a lack of standardization in the analytical process and high costs, which are still too high for large-scale use.
Keywords: biomarker; exosome; prostate cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

