In Vitro Assessment of the Synergistic Effect of Aspirin and 5-Fluorouracil in Colorectal Adenocarcinoma Cells
- PMID: 37504320
- PMCID: PMC10377900
- DOI: 10.3390/curroncol30070460
In Vitro Assessment of the Synergistic Effect of Aspirin and 5-Fluorouracil in Colorectal Adenocarcinoma Cells
Abstract
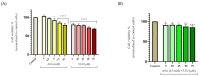
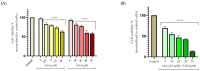
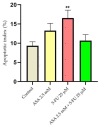
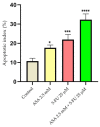
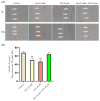
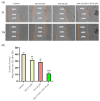
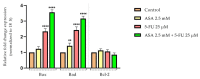
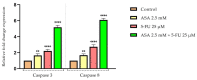
Although remarkable progress has been made, colorectal cancer remains a significant global health issue. One of the most challenging aspects of cancer treatment is the resistance of tumor cells to classical chemotherapy. Conventional therapy for colorectal cancer often involves the use of 5-fluorouracil as a chemotherapeutic agent. Aspirin, a drug used primarily to prevent cardiovascular complications, became a focus of attention due to its potential use as an antitumor agent. The purpose of the study was to evaluate the potential synergistic cytotoxic effects of aspirin and 5-fluorouracil on colorectal adenocarcinoma cells. The viability of cells, the impact on the morphology and nuclei of cells, the potential antimigratory effect, and the impact on the expression of the major genes associated with cell apoptosis (Bcl-2, Bax, Bad), as well as caspases 3 and 8, were evaluated. The results indicated that the two compounds exerted a synergistic effect, causing a reduction in cell viability accompanied by changes characteristic of the apoptosis process-the condensation of nuclei and the reorganization of actin filaments in cells, the reduction in the expression of the Bcl-2 gene, and the increase in the expression of Bax and Bad genes, along with caspases 3 and 8. Considering all these findings, it appears that aspirin may be investigated in depth in order to be used in conjunction with 5-fluorouracil to increase antitumor activity.
Keywords: 5-fluorouracil; aspirin; bcl-2 proteins; caspases; colorectal adenocarcinoma; immunofluorescence staining.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Hossain S., Karuniawati H., Jairoun A.A., Urbi Z., Ooi D.J., John A., Lim Y.C., Kibria K.M.K., Mohiuddin A.M., Ming L.C., et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers. 2022;14:1732. doi: 10.3390/cancers14071732. - DOI - PMC - PubMed
-
- Triantafillidis J.K., Nasioulas G., Kosmidis P.A. Colorectal cancer and inflammatory bowel disease: Epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer. Res. 2009;29:2727–2737. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

