A Review of Patents and Innovative Biopolymer-Based Hydrogels
- PMID: 37504436
- PMCID: PMC10378757
- DOI: 10.3390/gels9070556
A Review of Patents and Innovative Biopolymer-Based Hydrogels
Abstract
Biopolymers represent a great resource for the development and utilization of new functional materials due to their particular advantages such as biocompatibility, biodegradability and non-toxicity. "Intelligent gels" sensitive to different stimuli (temperature, pH, ionic strength) have different applications in many industries (e.g., pharmacy, biomedicine, food). This review summarizes the research efforts presented in the patent and non-patent literature. A discussion was conducted regarding biopolymer-based hydrogels such as natural proteins (i.e., fibrin, silk fibroin, collagen, keratin, gelatin) and polysaccharides (i.e., chitosan, hyaluronic acid, cellulose, carrageenan, alginate). In this analysis, the latest advances in the modification and characterization of advanced biopolymeric formulations and their state-of-the-art administration in drug delivery, wound healing, tissue engineering and regenerative medicine were addressed.
Keywords: alginate; carrageenan; cellulose; chitosan; collagen; fibrin; gelatin; hyaluronic acid; keratin; silk fibroin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
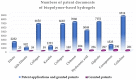





References
-
- Čolnik M., Knez-Hrnčič M., Škerget M., Knez Ž. Biodegradable polymers, current trends of research and their applications, a review. Chem. Ind. Chem. Eng. Q. 2020;26:401–418. doi: 10.2298/CICEQ191210018C. - DOI
-
- Saha S., Arshad M., Zubair M., Ullah A. Keratin as a Biopolymer. In: Sharma S., Kumar A., editors. Keratin as a Protein Biopolymer. Springer Nature; Basel, Switzerland: 2019. pp. 163–185. (Springer Series, on Polymer and Composite Materials).
-
- Fatimi A. Patentability of Biopolymer-Based Hydrogels. Chem. Proc. 2022;8:39.
Publication types
LinkOut - more resources
Full Text Sources

