Development and Comparative Evaluation of Ciprofloxacin Nanoemulsion-Loaded Bigels Prepared Using Different Ratios of Oleogel to Hydrogels
- PMID: 37504471
- PMCID: PMC10379317
- DOI: 10.3390/gels9070592
Development and Comparative Evaluation of Ciprofloxacin Nanoemulsion-Loaded Bigels Prepared Using Different Ratios of Oleogel to Hydrogels
Abstract
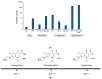
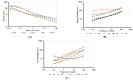
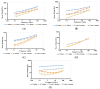
Nanoemulsions and bigels are biphasic delivery systems that can be used for topical applications. The aim of this study was to incorporate an oil-in-water ciprofloxacin hydrochloride nanoemulsion (CIP.HCl NE) into two types of bigels, Type I (oleogel (OL)-in-hydrogel (WH)) and Type II (WH-in-OL) to enhance drug penetration into skin and treat topical bacterial infections. Bigels were prepared at various ratios of OL and WH (1:1, 1:2, and 1:4). Initially, CIP.HCl NE was prepared and characterized in terms of droplet size, zeta potential, polydispersity index, morphology, and thermodynamic and chemical stability. Then CIP.HCl NE was dispersed into the OL or WH phase of the bigel. The primary physical stability studies showed that Type I bigels were physically stable, showing no phase separation. Whereas Type II bigels were physically unstable, hence excluded from the study. Type I bigels were subjected to microstructural, rheological, in vitro release, antimicrobial, and stability studies. The microscopic images showed a highly structured bigel network with nanoemulsion droplets dispersed within the bigel network. Additionally, bigels exhibited pseudoplastic flow and viscoelastic properties. A complete drug release was achieved after 4-5 h. The in vitro and ex vivo antimicrobial studies revealed that bigels exhibited antimicrobial activity against different bacterial strains. Moreover, stability studies showed that the rheological properties and physical and chemical stability varied based on the bigel composition over three months. Therefore, the physicochemical and rheological properties, drug release rate, and antimicrobial activity of Type I bigels could be modified by altering the OL to WH ratio and the phase in which the nanoemulsion dispersed in.
Keywords: antimicrobial activity; ciprofloxacin; hydrogel-in-oleogel bigel; nanoemulsions; oleogel-in-hydrogel bigel; rheology; topical delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Kahraman E., Kaykın M., Şahin Bektay H., Güngör S. Recent advances on topical application of ceramides to restore barrier function of skin. Cosmetics. 2019;6:52. doi: 10.3390/cosmetics6030052. - DOI
-
- Souto E.B., Fangueiro J.F., Fernandes A.R., Cano A., Sanchez-Lopez E., Garcia M.L., Severino P., Paganelli M.O., Chaud M.V., Silva A.M. Physicochemical and biopharmaceutical aspects influencing skin permeation and role of SLN and NLC for skin drug delivery. Heliyon. 2022;8:e08938. doi: 10.1016/j.heliyon.2022.e08938. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

