Performance-Enhancing Materials in Medical Gloves
- PMID: 37504844
- PMCID: PMC10381443
- DOI: 10.3390/jfb14070349
Performance-Enhancing Materials in Medical Gloves
Abstract
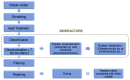
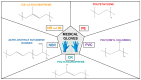
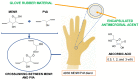
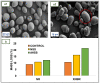
Medical gloves, along with masks and gowns, serve as the initial line of defense against potentially infectious microorganisms and hazardous substances in the health sector. During the COVID-19 pandemic, medical gloves played a significant role, as they were widely utilized throughout society in daily activities as a preventive measure. These products demonstrated their value as important personal protection equipment (PPE) and reaffirmed their relevance as infection prevention tools. This review describes the evolution of medical gloves since the discovery of vulcanization by Charles Goodyear in 1839, which fostered the development of this industry. Regarding the current market, a comparison of the main properties, benefits, and drawbacks of the most widespread types of sanitary gloves is presented. The most common gloves are produced from natural rubber (NR), polyisoprene (IR), acrylonitrile butadiene rubber (NBR), polychloroprene (CR), polyethylene (PE), and poly(vinyl chloride) (PVC). Furthermore, the environmental impacts of the conventional natural rubber glove manufacturing process and mitigation strategies, such as bioremediation and rubber recycling, are addressed. In order to create new medical gloves with improved properties, several biopolymers (e.g., poly(vinyl alcohol) and starch) and additives such as biodegradable fillers (e.g., cellulose and chitin), reinforcing fillers (e.g., silica and cellulose nanocrystals), and antimicrobial agents (e.g., biguanides and quaternary ammonium salts) have been evaluated. This paper covers these performance-enhancing materials and describes different innovative prototypes of gloves and coatings designed with them.
Keywords: antimicrobial properties; bio-filler; medical gloves; natural rubber; performance-enhancing materials; reinforcing filler; synthetic rubber.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
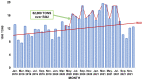
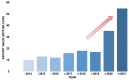
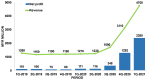
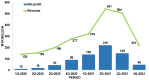







References
-
- Mazón L., Orriols R.M. Sanitary Gloves Management. Adequate Protection-Effectiveness and Environmental Responsibility. Rev. Asoc. Española Espec. Med. Trab. 2018;27:175–181.
-
- Delves P.J., Martin S.J., Burton D.R., Roitt I.M. Roitt’s Essential Immunology. 13th ed. John Wiley and Sons; Hoboken, NJ, USA: 2017.
-
- Babadi A.A., Bagheri S., Hamid S.B.A. Progress on Antimicrobial Surgical Gloves: A Review. Rubber Chem. Technol. 2016;89:117–125. doi: 10.5254/rct.15.84882. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

