Effect of Chitosan on Rheological, Mechanical, and Adhesive Properties of Pectin-Calcium Gel
- PMID: 37504906
- PMCID: PMC10381555
- DOI: 10.3390/md21070375
Effect of Chitosan on Rheological, Mechanical, and Adhesive Properties of Pectin-Calcium Gel
Abstract
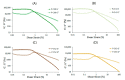
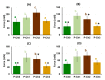
In the present study, chitosan was included in the pectin ionotropic gel to improve its mechanical and bioadhesive properties. Pectin-chitosan gels P-Ch0, P-Ch1, P-Ch2, and P-Ch3 of chitosan weight fractions of 0.00, 0.25, 0.50, and 0.75 were prepared and characterized by dynamic rheological tests, penetration tests, and serosal adhesion ex vivo assays. The storage modulus (G') and loss modulus (G″) values, gel hardness, and elasticity of P-Ch1 were significantly higher than those of P-Ch0 gel. However, a further increase in the content of chitosan in the gel significantly reduced these parameters. The inclusion of chitosan into the pectin gel led to a decrease in weight and an increase in hardness during incubation in Hanks' solution at pH 5.0, 7.4, and 8.0. The adhesion of P-Ch1 and P-Ch2 to rat intestinal serosa ex vivo was 1.3 and 1.7 times stronger, whereas that of P-Ch3 was similar to that of a P-Ch0 gel. Pre-incubation in Hanks' solution at pH 5.0 and 7.4 reduced the adhesivity of gels; however, the adhesivity of P-Ch1 and P-Ch2 exceeded that of P-Ch0 and P-Ch3. Thus, serosal adhesion combined with higher mechanical stability in a wide pH range appeared to be advantages of the inclusion of chitosan into pectin gel.
Keywords: chitosan; ionotropic gel; mechanical properties; pectin; rheology; serosal adhesion; weight loss.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






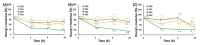






Similar articles
-
Serosal Adhesion Ex Vivo of Hydrogels Prepared from Apple Pectin Cross-Linked with Fe3+ Ions.Int J Mol Sci. 2023 Jan 8;24(2):1248. doi: 10.3390/ijms24021248. Int J Mol Sci. 2023. PMID: 36674765 Free PMC article.
-
Microstructure and kinetic rheological behavior of amidated and nonamidated LM pectin gels.Biomacromolecules. 2006 Jan;7(1):114-21. doi: 10.1021/bm050459r. Biomacromolecules. 2006. PMID: 16398505
-
Characterization of microstructure, viscoelasticity, heterogeneity and ergodicity in pectin-laponite-CTAB-calcium nanocomposite hydrogels.Carbohydr Polym. 2016 Jan 20;136:242-9. doi: 10.1016/j.carbpol.2015.09.031. Epub 2015 Sep 11. Carbohydr Polym. 2016. PMID: 26572352
-
Effects of calcium, pH, and blockiness on kinetic rheological behavior and microstructure of HM pectin gels.Biomacromolecules. 2005 Mar-Apr;6(2):646-52. doi: 10.1021/bm049619+. Biomacromolecules. 2005. PMID: 15762625
-
Pectin as a rheology modifier: Origin, structure, commercial production and rheology.Carbohydr Polym. 2017 Apr 1;161:118-139. doi: 10.1016/j.carbpol.2016.12.033. Epub 2016 Dec 24. Carbohydr Polym. 2017. PMID: 28189220 Review.
Cited by
-
New Antimicrobial Gels Based on Clove Essential Oil-Cyclodextrin Complex and Plant Extracts for Topical Use.Gels. 2025 Aug 18;11(8):653. doi: 10.3390/gels11080653. Gels. 2025. PMID: 40868783 Free PMC article.
-
Swelling, Protein Adsorption, and Biocompatibility of Pectin-Chitosan Hydrogels.Gels. 2024 Jul 17;10(7):472. doi: 10.3390/gels10070472. Gels. 2024. PMID: 39057495 Free PMC article.
References
-
- Phillips B. Reducing gastrointestinal anastomotic leak rates: Review of challenges and solutions. Open Access Surg. 2016;9:5–14. doi: 10.2147/OAS.S54936. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

