Continuous Flow Chemistry: A Novel Technology for the Synthesis of Marine Drugs
- PMID: 37504932
- PMCID: PMC10381277
- DOI: 10.3390/md21070402
Continuous Flow Chemistry: A Novel Technology for the Synthesis of Marine Drugs
Abstract
In this perspective, we showcase the benefits of continuous flow chemistry and photochemistry and how these valuable tools have contributed to the synthesis of organic scaffolds from the marine environment. These technologies have not only facilitated previously described synthetic pathways, but also opened new opportunities in the preparation of novel organic molecules with remarkable pharmacological properties which can be used in drug discovery programs.
Keywords: drug discovery; flow chemistry; marine drugs; novel technologies; photochemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




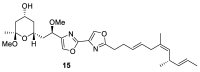


















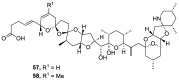

References
-
- Alcázar J. Sustainable Flow Chemistry. Wiley; Hoboken, NJ, USA: 2017. Sustainable Flow Chemistry in Drug Discovery; pp. 135–164.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

