Molecular, Cellular, and Functional Heterogeneity of Retinal and Choroidal Endothelial Cells
- PMID: 37504962
- PMCID: PMC10382998
- DOI: 10.1167/iovs.64.10.35
Molecular, Cellular, and Functional Heterogeneity of Retinal and Choroidal Endothelial Cells
Abstract
Purpose: To investigate the endothelial heterogeneity across distinct vascular beds in the inner and outer blood-retinal barriers.
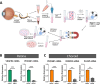
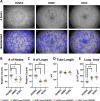
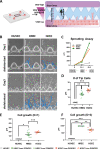
Methods: We evaluated the molecular, cellular, and functional differences between primary human retinal endothelial cells (HRECs) and human choroidal endothelial cells (HCECs) in terms of angiogenic and vasculogenic properties, permeability, and transcytosis. Tube formation assay, cell migration assay, in vitro permeability assay, microfluidic sprouting assay, and transcriptome analysis were performed.
Results: HRECs showed higher proliferation and migration activity than did HCECs, whereas the tube formation ability was similar between HRECs and HCECs. Under angiogenic stimuli, HCECs displayed earlier sprouting angiogenesis, but the overall speed was faster and more stable in HRECs. HRECs expressed higher levels of adherens junctional proteins, whereas the tight junctional genes and transcytosis-related genes were more highly expressed in HCECs. Angiopoietin-2 was predominantly expressed in HRECs, but vascular endothelial growth factor (VEGF) receptors were more strongly expressed in HCECs. Platelet-derived growth factor subunit B (PDGFB) was more highly expressed in HRECs, which correlates to the lower degree of pericyte coverage in choroidal blood vessels.
Conclusions: Retinal and choroidal ECs showed significant cellular and molecular heterogeneities that correlated with their functional characteristics. Retinal ECs are vasculogenic with high migratory characteristics and faster angiogenic sprouting, and they are more responsive to VEGF-induced permeability. In contrast, choroidal ECs express high levels of transcytosis genes, and they are vasculogenic, rather proliferative, adept in generating tip cells, and less responsive to VEGF-induced permeability.
Conflict of interest statement
Disclosure:
Figures




References
-
- Augustin HG, Koh GY.. Organotypic vasculature: from descriptive heterogeneity to functional pathophysiology. Science. 2017; 357: eaal2379. - PubMed
-
- Anand-Apte B, Hollyfield JG.. Developmental anatomy of the retinal and choroidal vasculature. In: Dartt DA, ed. Encyclopedia of the Eye . Oxford: Academic Press; 2010: 9–15.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

