Phosphates Transfer in Pristine and Modified CJMA-2 Membrane during Electrodialysis Processing of NaxH(3-x)PO4 Solutions with pH from 4.5 to 9.9
- PMID: 37505013
- PMCID: PMC10386648
- DOI: 10.3390/membranes13070647
Phosphates Transfer in Pristine and Modified CJMA-2 Membrane during Electrodialysis Processing of NaxH(3-x)PO4 Solutions with pH from 4.5 to 9.9
Abstract
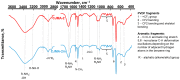
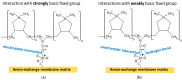
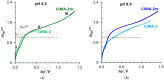
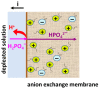
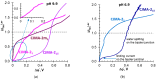
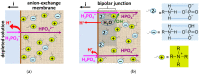
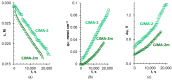
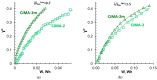
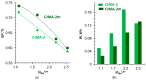
Phosphate recovery from different second streams using electrodialysis (ED) is a promising step to a nutrients circular economy. However, the relatively low ED performance hinders the widespread adoption of this environmentally sound method. The formation of "bonded species" between phosphates and the weakly basic fixed groups (primary and secondary amines) of the anion exchange membrane can be the cause of decrease in current efficiency and increase in energy consumption. ED processing of NaxH(3-x)PO4 alkaline solutions and the use of intense current modes promote the formation of a bipolar junction from negatively charged bound species and positively charged fixed groups. This phenomenon causes a change in the shape of current-voltage curves, increase in resistance, and an enhancement in proton generation during long-term operation of anion-exchange membrane with weakly basic fixed groups. Shielding of primary and secondary amines with a modifier containing quaternary ammonium bases significantly improves ED performance in the recovery of phosphates from NaxH(3-x)PO4 solution with pH 4.5. Indeed, in the limiting and underlimiting current modes, 40% of phosphates are recovered 1.3 times faster, and energy consumption is reduced by 1.9 times in the case of the modified membrane compared to the pristine one. Studies were performed using a new commercial anion exchange membrane CJMA-2.
Keywords: anion exchange membrane; bound species; boundary junction; current efficiency; current–voltage curve; electrodialysis; energy consumption; phosphates transfer; proton generation; weakly basic fixed groups.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
How Chemical Nature of Fixed Groups of Anion-Exchange Membranes Affects the Performance of Electrodialysis of Phosphate-Containing Solutions?Polymers (Basel). 2023 May 12;15(10):2288. doi: 10.3390/polym15102288. Polymers (Basel). 2023. PMID: 37242863 Free PMC article.
-
Effect of Pulsed Electric Field on the Electrodialysis Performance of Phosphate-Containing Solutions.Membranes (Basel). 2022 Nov 5;12(11):1107. doi: 10.3390/membranes12111107. Membranes (Basel). 2022. PMID: 36363662 Free PMC article.
-
Characterization of MK-40 Membrane Modified by Layers of Cation Exchange and Anion Exchange Polyelectrolytes.Membranes (Basel). 2020 Jan 27;10(2):20. doi: 10.3390/membranes10020020. Membranes (Basel). 2020. PMID: 32012783 Free PMC article.
-
Recovery of Nutrients from Residual Streams Using Ion-Exchange Membranes: Current State, Bottlenecks, Fundamentals and Innovations.Membranes (Basel). 2022 May 4;12(5):497. doi: 10.3390/membranes12050497. Membranes (Basel). 2022. PMID: 35629823 Free PMC article. Review.
-
Surface Modifications of Anion Exchange Membranes for an Improved Reverse Electrodialysis Process Performance: A Review.Membranes (Basel). 2020 Jul 22;10(8):160. doi: 10.3390/membranes10080160. Membranes (Basel). 2020. PMID: 32707798 Free PMC article. Review.
Cited by
-
Phosphate Transport Through Homogeneous and Heterogeneous Anion-Exchange Membranes: A Chronopotentiometric Study for Electrodialytic Applications.Membranes (Basel). 2025 Jul 31;15(8):230. doi: 10.3390/membranes15080230. Membranes (Basel). 2025. PMID: 40863591 Free PMC article.
References
-
- Knežević K., Saracevic E., Krampe J., Kreuzinger N. Comparison of ion removal from waste fermentation effluent by nanofiltration, electrodialysis and ion exchange for a subsequent sulfuric acid recovery. J. Environ. Chem. Eng. 2022;10:108423. doi: 10.1016/j.jece.2022.108423. - DOI
-
- Wang Q., Chen G.Q., Lin L., Li X., Kentish S.E. Purification of organic acids using electrodialysis with bipolar membranes (EDBM) combined with monovalent anion selective membranes. Sep. Purif. Technol. 2021;279:119739. doi: 10.1016/j.seppur.2021.119739. - DOI
-
- Hülber-Beyer É., Bélafi-Bakó K., Nemestóthy N. Low-waste fermentation-derived organic acid production by bipolar membrane electrodialysis—An overview. Chem. Pap. 2021;75:5223–5234. doi: 10.1007/s11696-021-01720-w. - DOI
-
- Wang Z., He P., Zhang H., Zhang N., Lü F. Desalination, nutrients recovery, or products extraction: Is electrodialysis an effective way to achieve high-value utilization of liquid digestate? Chem. Eng. J. 2022;446:136996. doi: 10.1016/j.cej.2022.136996. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

