Electrodeposited Ionomer Protection Layer for Negative Electrodes in Zinc-Air Batteries
- PMID: 37505046
- PMCID: PMC10385867
- DOI: 10.3390/membranes13070680
Electrodeposited Ionomer Protection Layer for Negative Electrodes in Zinc-Air Batteries
Abstract
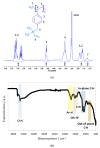
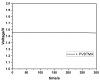
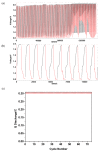
The protection of zinc anodes in zinc-air batteries (ZABs) is an efficient way to reduce corrosion and Zn dendrite formation and improve cyclability and battery efficiency. Anion-conducting poly(N-vinylbenzyl N,N,N-trimethylammonium)chloride (PVBTMA) thin films were electrodeposited directly on zinc metal using cyclic voltammetry. This deposition process presents a combination of advantages, including selective anion transport in PVBTMA reducing zinc crossover, high interface quality by electrodeposition improving the corrosion protection of zinc and high ionomer stiffness opposing zinc dendrite perforation. The PVBTMA layer was observed by optical and electron microscopy, and the wettability of the ionomer-coated surface was investigated by contact angle measurements. ZABs with PVBTMA-coated Zn showed an appreciable and stable open-circuit voltage both in alkaline electrolyte (1.55 V with a Pt cathode) and in miniaturized batteries (1.31 V with a carbon paper cathode). Cycling tests at 0.5 mA/cm2 within voltage limits of 2.1 and 0.8 V gave a stable discharge capacity for nearly 100 cycles with a liquid electrolyte and more than 20 cycles in miniaturized batteries. The faster degradation of the latter ZAB was attributed to the clogging of the carbon air cathode and drying or carbonation of the electrolyte sorbed in a Whatman paper.
Keywords: anion exchange membranes; electropolymerization; metal–air batteries; oxygen reduction reaction.
Conflict of interest statement
There are no conflict to declare.
Figures












References
-
- Chakkaravarthy C., Waheed A.K.A., Udupa H.V.K. Zinc-air alkaline batteries—A review. J. Power Sources. 1981;6:203–228. doi: 10.1016/0378-7753(81)80027-4. - DOI
-
- Caramia V., Bozzini B. Materials science aspects of zinc–air batteries: A review. Mater. Renew. Sustain. Energy. 2014;3:28. doi: 10.1007/s40243-014-0028-3. - DOI
LinkOut - more resources
Full Text Sources

