The constructive and destructive impact of autophagy on both genders' reproducibility, a comprehensive review
- PMID: 37505071
- PMCID: PMC10621263
- DOI: 10.1080/15548627.2023.2238577
The constructive and destructive impact of autophagy on both genders' reproducibility, a comprehensive review
Erratum in
-
Correction.Autophagy. 2025 Jun;21(6):iv-vi. doi: 10.1080/15548627.2023.2257071. Epub 2023 Sep 13. Autophagy. 2025. PMID: 37700720 Free PMC article. No abstract available.
Abstract
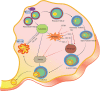
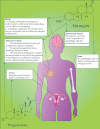
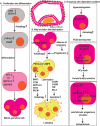
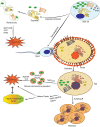
Reproduction is characterized by a series of massive renovations at molecular, cellular, and tissue levels. Recent studies have strongly tended to reveal the involvement of basic molecular pathways such as autophagy, a highly conserved eukaryotic cellular recycling, during reproductive processes. This review comprehensively describes the current knowledge, updated to September 2022, of autophagy contribution during reproductive processes in males including spermatogenesis, sperm motility and viability, and male sex hormones and females including germ cells and oocytes viability, ovulation, implantation, fertilization, and female sex hormones. Furthermore, the consequences of disruption in autophagic flux on the reproductive disorders including oligospermia, azoospermia, asthenozoospermia, teratozoospermia, globozoospermia, premature ovarian insufficiency, polycystic ovarian syndrome, endometriosis, and other disorders related to infertility are discussed as well.Abbreviations: AKT/protein kinase B: AKT serine/threonine kinase; AMPK: AMP-activated protein kinase; ATG: autophagy related; E2: estrogen; EDs: endocrine disruptors; ER: endoplasmic reticulum; FSH: follicle stimulating hormone; FOX: forkhead box; GCs: granulosa cells; HIF: hypoxia inducible factor; IVF: in vitro fertilization; IVM: in vitro maturation; LCs: Leydig cells; LDs: lipid droplets; LH: luteinizing hormone; LRWD1: leucine rich repeats and WD repeat domain containing 1; MAP1LC3: microtubule associated protein 1 light chain 3; MAPK: mitogen-activated protein kinase; MTOR: mechanistic target of rapamycin kinase; NFKB/NF-kB: nuclear factor kappa B; P4: progesterone; PCOS: polycystic ovarian syndrome; PDLIM1: PDZ and LIM domain 1; PI3K: phosphoinositide 3-kinase; PtdIns3P: phosphatidylinositol-3-phosphate; PtdIns3K: class III phosphatidylinositol 3-kinase; POI: premature ovarian insufficiency; ROS: reactive oxygen species; SCs: Sertoli cells; SQSTM1/p62: sequestosome 1; TSGA10: testis specific 10; TST: testosterone; VCP: vasolin containing protein.
Keywords: Apoptosis; autophagy; fertility; follicles; granulosa cells; infertility.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
