Macrocyclic Peptidomimetic Plasmepsin X Inhibitors with Potent In Vitro and In Vivo Antimalarial Activity
- PMID: 37505188
- PMCID: PMC10424242
- DOI: 10.1021/acs.jmedchem.3c00812
Macrocyclic Peptidomimetic Plasmepsin X Inhibitors with Potent In Vitro and In Vivo Antimalarial Activity
Abstract
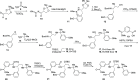
The Plasmodium falciparum aspartic protease plasmepsin X (PMX) is essential for the egress of invasive merozoite forms of the parasite. PMX has therefore emerged as a new potential antimalarial target. Building on peptidic amino alcohols originating from a phenotypic screening hit, we have here developed a series of macrocyclic analogues as PMX inhibitors. Incorporation of an extended linker between the S1 phenyl group and S3 amide led to a lead compound that displayed a 10-fold improved PMX inhibitory potency and a 3-fold improved half-life in microsomal stability assays compared to the acyclic analogue. The lead compound was also the most potent of the new macrocyclic compounds in in vitro parasite growth inhibition. Inhibitor 7k cleared blood-stage P. falciparum in a dose-dependent manner when administered orally to infected humanized mice. Consequently, lead compound 7k represents a promising orally bioavailable molecule for further development as a PMX-targeting antimalarial drug.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
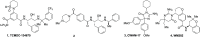
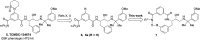
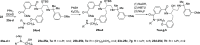
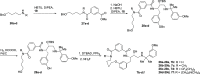
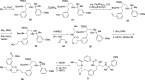
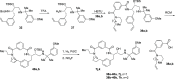
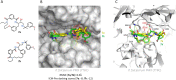
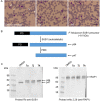
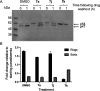
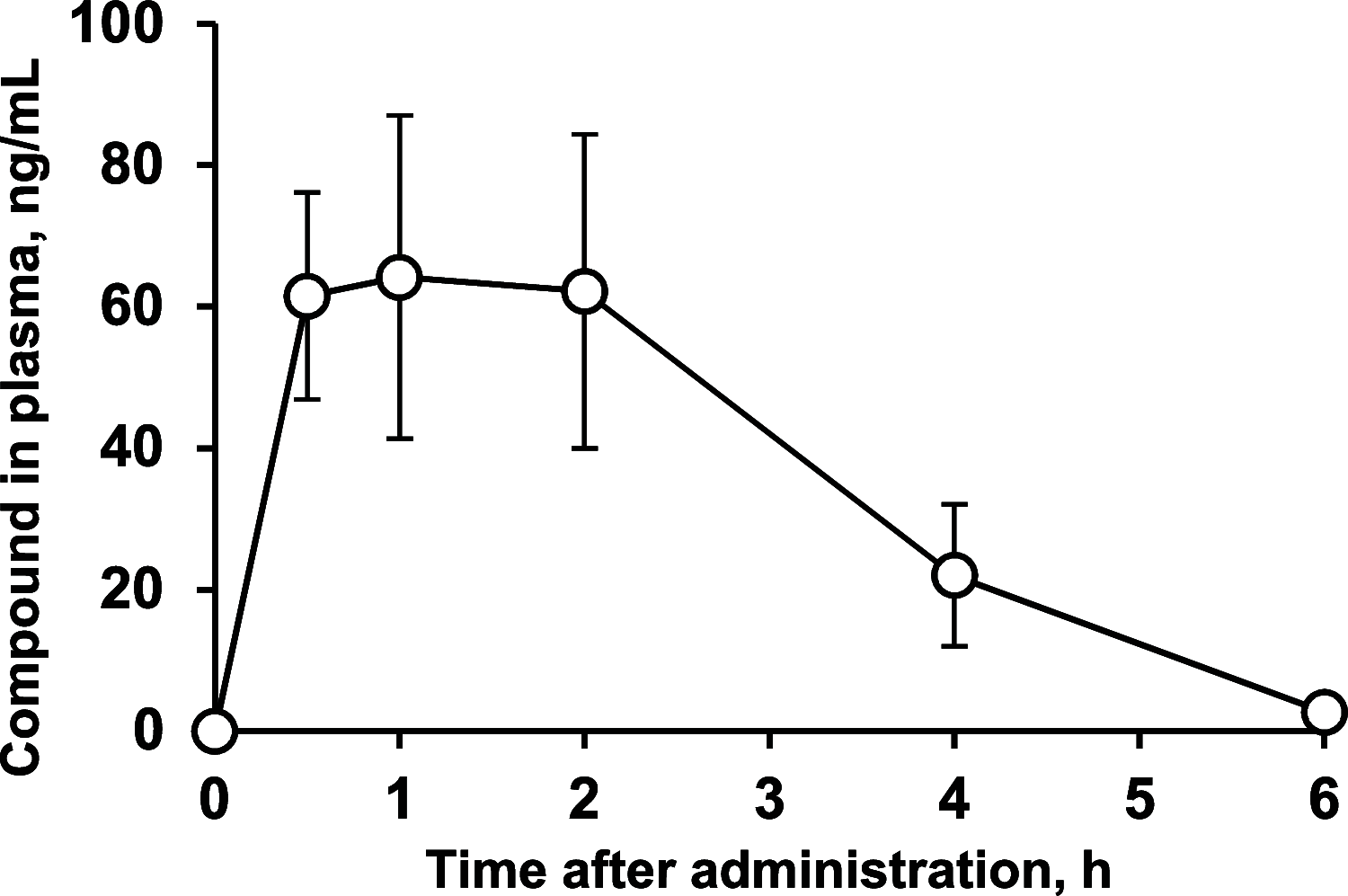

References
-
- WHO, World Malaria Report 2022, World Health Organ: Geneva, 2016. https://www.who.int/publications/i/item/9789240064898.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information

