Genotyping of Trypanosoma brucei evansi in Egyptian camels: detection of a different non-RoTat 1.2 Trypanosoma brucei evansi in Egyptian camels
- PMID: 37505344
- PMCID: PMC10382407
- DOI: 10.1007/s11250-023-03673-6
Genotyping of Trypanosoma brucei evansi in Egyptian camels: detection of a different non-RoTat 1.2 Trypanosoma brucei evansi in Egyptian camels
Abstract
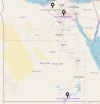
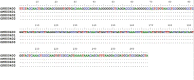
Trypanosoma brucei evansi (T. b. evansi) is an enzootic organism found in Egyptian camels, which genetically classified into types A and B. To detect the parasite genotype circulating in Egyptian camels, we collected 94 blood samples from three distant districts and subjected them to different PCR assays; T. brucei repeat (TBR), internal transcribed spacer-1 (ITS-1), and variable surface glycoproteins (VSG) (RoTat 1. 2, JN 2118Hu) and EVAB PCRs. The highest prevalence was obtained with TBR (80/91; 87.9%), followed by ITS-1 (52/91; 57.1%), VSG JN 2118Hu (42/91; 46.2%), and VSG RoTat 1. 2 (34/91; 37.4%). We reported a different non-RoTat 1. 2 T. b. evansi for the first time in Egyptian camels. Results showed that 47 (58.7%) out of 80 samples were classified as T. b. evansi. Of these, 14 (29.8%) were RoTat 1. 2 type, 13 (27.6%) were non-RoTat 1. 2 type, and 20 (42.6%) samples were from mixed infection with both types. All samples were tested negative with EVAB PCR. RoTat 1. 2 T. b. evansi was the most prevalent in Giza and El Nubariyah, whereas, in Aswan, the only type detected was non-RoTat 1. 2 T. b. evansi. The nucleotide sequences of the VSG RoTat 1.2 and JN 2118Hu PCR products were submitted to DNA Data Bank of Japan (DDBJ) and GenBank under the accession numbers LC738852, and (OP800400-OP800403). Further research is required to increase the sample size and verify the new sequences to corroborate the prevalence of a new variant of non-RoTat 1.2 T. b. evansi in Egypt.
Keywords: EVAB PCR; Egyptian camels; ITS-1 PCR; JN 2118Hu PCR; Non-RoTat1.2; T. B. evansi; TBR PCR; VSG RoTat 1.2 PCR.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Parasitological and molecular investigation of Trypanosoma evansi in dromedaries from Greater Cairo, Egypt.J Vet Med Sci. 2024 Nov 15;86(11):1177-1184. doi: 10.1292/jvms.24-0284. Epub 2024 Oct 2. J Vet Med Sci. 2024. PMID: 39358244 Free PMC article.
-
Molecular survey and characterization of Trypanosoma evansi in naturally infected camels with suspicion of a Trypanozoon infection in horses by molecular detection in Egypt.Microb Pathog. 2018 Oct;123:201-205. doi: 10.1016/j.micpath.2018.07.017. Epub 2018 Jul 18. Microb Pathog. 2018. PMID: 30016680
-
PCR amplification of RoTat 1.2 VSG gene in Trypanosoma evansi isolates in Kenya.Vet Parasitol. 2004 Feb 26;120(1-2):23-33. doi: 10.1016/j.vetpar.2003.12.007. Vet Parasitol. 2004. PMID: 15019140
-
The detection of non-RoTat 1.2 Trypanosoma evansi.Exp Parasitol. 2005 May;110(1):30-8. doi: 10.1016/j.exppara.2005.01.001. Exp Parasitol. 2005. PMID: 15804376
-
Molecular and parasitological detection of Trypanosoma evansi in Camels in Ismailia, Egypt.Vet Parasitol. 2013 Nov 15;198(1-2):214-8. doi: 10.1016/j.vetpar.2013.08.003. Epub 2013 Aug 14. Vet Parasitol. 2013. PMID: 24029715
Cited by
-
Recent Progress in the Detection of Surra, a Neglected Disease Caused by Trypanosoma evansi with a One Health Impact in Large Parts of the Tropic and Sub-Tropic World.Microorganisms. 2023 Dec 26;12(1):44. doi: 10.3390/microorganisms12010044. Microorganisms. 2023. PMID: 38257871 Free PMC article. Review.
-
Molecular identification of new Trypanosoma evansi type non-A/B isolates from buffaloes and cattle in Indonesia.Rev Bras Parasitol Vet. 2024 Jun 28;33(2):e001324. doi: 10.1590/S1984-29612024033. eCollection 2024. Rev Bras Parasitol Vet. 2024. PMID: 38958293 Free PMC article.
-
Parasitological and molecular investigation of Trypanosoma evansi in dromedaries from Greater Cairo, Egypt.J Vet Med Sci. 2024 Nov 15;86(11):1177-1184. doi: 10.1292/jvms.24-0284. Epub 2024 Oct 2. J Vet Med Sci. 2024. PMID: 39358244 Free PMC article.
-
Impact of trypanosomiasis on male camel infertility.Front Vet Sci. 2025 Jan 16;11:1506532. doi: 10.3389/fvets.2024.1506532. eCollection 2024. Front Vet Sci. 2025. PMID: 39885842 Free PMC article.
-
A CRISPR-Cas-based recombinase polymerase amplification assay for ultra-sensitive detection of active Trypanosoma brucei evansi infections.Front Mol Biosci. 2025 Feb 14;12:1512970. doi: 10.3389/fmolb.2025.1512970. eCollection 2025. Front Mol Biosci. 2025. PMID: 40026698 Free PMC article.
References
-
- Al-Kharusi A, Elshafie EI, Baqir S, Faraz A, Al-Ansari A, Burger P, Mahgoub O, Al-Kharousi K, Al-Duhli H, Al-Sinani M, Al-Hatali R, Roberts D. Detection of Trypanosoma Infection in Dromedary Camels by Using Different Diagnostic Techniques in Northern Oman. Animals. 2022;12:1348. doi: 10.3390/ani12111348. - DOI - PMC - PubMed
-
- Birhanu H, Fikru R, Mussa S, Weldu K, Tadesse G, Hago A, Alemu T, Dawit T, BerKvens D, Goddeeris BM, Bűscher P. Epidemiology of Trypanosoma evansi and Trypanosoma vivax in domestic animals from selected districts of Tigray and Afar regions, Northern Ethiopia. Parasites and Vectors. 2015;8:212. doi: 10.1186/s13071-015-0818-1. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

