Liposomal nanocarriers of preassembled glycocalyx expeditiously restore endothelial glycocalyx in endotoxemia
- PMID: 37505471
- PMCID: PMC10643000
- DOI: 10.1152/ajpheart.00196.2023
Liposomal nanocarriers of preassembled glycocalyx expeditiously restore endothelial glycocalyx in endotoxemia
Abstract
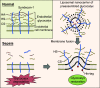
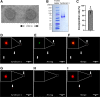
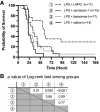
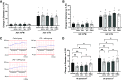
The endothelial glycocalyx (EG) is degraded early during sepsis, and currently available treatments are not effective in promptly restoring it. Here, we created liposomal nanocarriers of preassembled glycocalyx (LNPG) by synthesizing glycosylated syndecan-1 and inserting it into the lipid membrane of unilamellar liposomes. We hypothesized that LNPG would fuse with the endothelial cells where EG is degraded and restore EG in sepsis. We induced endotoxemia in C57BL/6J mice using lipopolysaccharides (LPS) and treated them with LNPG, saline, syndecan-1, or liposomes. LNPG significantly prolonged the survival time of LPS-treated mice compared with the other treatments. Immunostaining of en face mesenteric arteries of LPS-treated mice showed that syndecan-1 was fully restored after LNPG administration. In addition, EG height in microvasculature of mouse cremaster muscle was monitored using sidestream dark field imaging. LNPG restored the perfused boundary region (PBR), which is inversely related to EG dimensions, to the control level after LPS administration. Furthermore, flow-induced dilation in isolated mouse mesenteric arterioles was fully recovered after LNPG treatment in LPS-treated mice. In summary, our findings provide evidence of the therapeutic efficacy of LNPG in the LPS-induced mouse model of sepsis, achieved by expeditiously restoring EG through fusion of LNPG with the endothelial plasma membrane and recovery of endothelial function.NEW & NOTEWORTHY Vascular endothelial cells represent the first line of exposure to bacterial endotoxins. Here, we propose a novel therapeutic strategy using liposomes to deliver preassembled glycocalyx to vascular endothelial cell surface and consequently restore endothelial glycocalyx (EG). We tested liposomal nanocarriers of preassembled glycocalyx (LNPG) in vivo and ex vivo to establish for the first time their expeditious therapeutic efficacy in improving survival of lipopolysaccharides (LPS)-treated mice, as achieved by the restoration of EG and recovery of endothelial function.
Keywords: endotoxemia; glycocalyx; liposome; microcirculation; syndecan-1.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


Comment in
-
Patching up a degraded endothelial glycocalyx in sepsis.Am J Physiol Heart Circ Physiol. 2023 Oct 1;325(4):H673-H674. doi: 10.1152/ajpheart.00499.2023. Epub 2023 Aug 18. Am J Physiol Heart Circ Physiol. 2023. PMID: 37594482 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

