Degradation of Chemical Warfare Agent Nitrogen Mustard Using Ferrate (VI)
- PMID: 37505525
- PMCID: PMC10384491
- DOI: 10.3390/toxics11070559
Degradation of Chemical Warfare Agent Nitrogen Mustard Using Ferrate (VI)
Abstract
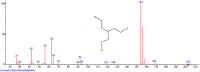
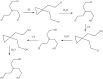
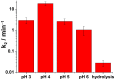
Chemical warfare agents (CWAs) are one of the most toxic compounds. Degradation of CWAs using decontamination agents is one of the few ways to protect human health against the harmful effects of CWAs. A ferrate (VI)-based potential chemical warfare agent decontaminant was studied for the degradation of persistent nitrogen mustard (tris(2-chloroethyl)amine, HN3). By optimizing the reaction conditions, the complete degradation of HN3 was achieved in 4 min. The degradation products contained mostly reduced Fe species, which confirmed the environmental friendliness of the proposed decontamination solution.
Keywords: chemical warfare agent; decontamination; ferrate (VI); nitrogen mustard.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Singh B., Prasad G.K., Pandey K.S., Danikhel R.K., Vijayaraghavan R. Decontamination of Chemical Warfare Agents. Def. Sci. J. 2010;60:428. doi: 10.14429/dsj.60.487. - DOI
-
- Gupta R.C. Handbook of Toxicology of Chemical Warfare Agents. Academic Press; Cambridge, MA, USA: 2015. no. BN.
Grants and funding
LinkOut - more resources
Full Text Sources

