Changes in Biomarkers of Exposure and Potential Harm in Smokers Switched to Vuse Vibe or Vuse Ciro Electronic Nicotine Delivery Systems
- PMID: 37505530
- PMCID: PMC10384956
- DOI: 10.3390/toxics11070564
Changes in Biomarkers of Exposure and Potential Harm in Smokers Switched to Vuse Vibe or Vuse Ciro Electronic Nicotine Delivery Systems
Abstract
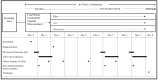
Electronic nicotine delivery systems (ENDS) have the potential to provide nicotine to tobacco consumers while reducing exposure to combustion-related toxicants. Here, we report changes in biomarkers of exposure (BoE) and biomarkers of potential harm (BoPH) in smokers who completely switched to Vuse Vibe and Vuse Ciro ENDS products, or to smoking abstinence in a randomized, controlled clinical study. Thirteen BoE (12 urinary and one blood) that indicate exposure to harmful and potentially harmful toxicants (HPHCs) were evaluated at baseline on day 5. Urinary BoPH linked to oxidative stress, platelet activation, and inflammation were also assessed at baseline, and on day 5 and day 7. Nicotine exposure was lower in Vuse Vibe and Vuse Ciro groups compared to baseline values. Urinary non-nicotine BoE decreased significantly (52.3-96.7%) in the Vuse ENDS groups, and the reductions were similar in magnitude to those observed in the abstinence group. Blood carboxyhemoglobin decreased 52.8-55.0% in all study groups. Decreases (10-50%) in BoPH were observed in all study groups. Thus, smokers who switch exclusively to Vuse Vibe or Vuse Ciro products or completely abstain from smoking are exposed to substantially lower levels of HPHCs, and experience improvements in BoPH of oxidative stress and inflammation pathways.
Keywords: BoE; BoPH; ENDS; abstinence; cigarette smokers; switching study.
Conflict of interest statement
M.N.K., P.M., P.C. and J.W.C. are full-time employees of RAI Services Company, E.K.R. is a full-time employee of BAT (Investments) Limited, and B.G.B., G.L.P. and P.R.N. are former full-time employees of RAI Services Company. G.L.P. works as an independent scientific consultant. RAI Services Company is a wholly owned subsidiary of Reynolds American Inc., which is an indirect, wholly owned subsidiary of British American Tobacco plc.
Figures

References
-
- Centers for Diseases Control and Prevention . How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Centers for Disease Control and Prevention; Atlanta, GA, USA: 2010. - PubMed
-
- Food and Drug Administration . US Department of Health and Human Services; Washington, DC, USA: 2012. [(accessed on 8 September 2022)]. Established List. Available online: https://www.govinfo.gov/content/pkg/FR-2012-04-03/pdf/2012-7727.pdf.
-
- United States Public Health Service Office of the Surgeon, General, Prevention National Center for Chronic Disease, Smoking Health Promotion Office On, and Health . Smoking Cessation: A Report of the Surgeon General. US Department of Health and Human Services; Washington, DC, USA: 2020. Publications and Reports of the Surgeon General.
-
- Institute of Medicine . Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction. The National Academies Press; Washington, DC, USA: 2001. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

