Time- and Concentration-Dependent Adverse Effects of Paclitaxel on Non-Neuronal Cells in Rat Primary Dorsal Root Ganglia
- PMID: 37505547
- PMCID: PMC10385404
- DOI: 10.3390/toxics11070581
Time- and Concentration-Dependent Adverse Effects of Paclitaxel on Non-Neuronal Cells in Rat Primary Dorsal Root Ganglia
Abstract
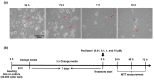
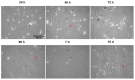
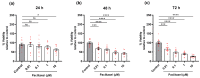
Paclitaxel is a chemotherapeutic agent used to treat a wide range of malignant tumors. Although it has anti-tumoral properties, paclitaxel also shows significant adverse effects on the peripheral nervous system, causing peripheral neuropathy. Paclitaxel has previously been shown to exert direct neurotoxic effects on primary DRG neurons. However, little is known about paclitaxel's effects on non-neuronal DRG cells. They provide mechanical and metabolic support and influence neuronal signaling. In the present study, paclitaxel effects on primary DRG non-neuronal cells were analyzed and their concentration or/and time dependence investigated. DRGs of Wister rats (6-8 weeks old) were isolated, and non-neuronal cell populations were separated by the density gradient centrifugation method. Different concentrations of Paclitaxel (0.01 µM-10 µM) were tested on cell viability by MTT assay, cell death by lactate dehydrogenase (LDH) assay, and propidium iodide (PI) assay, as well as cell proliferation by Bromodeoxyuridine (BrdU) assay at 24 h, 48 h, and 72 h post-treatment. Furthermore, phenotypic effects have been investigated by using immunofluorescence techniques. Paclitaxel exhibited several toxicological effects on non-neuronal cells, including a reduction in cell viability, an increase in cell death, and an inhibition of cell proliferation. These effects were concentration- and time-dependent. Cellular and nuclear changes such as shrinkage, swelling of cell bodies, nuclear condensation, chromatin fragmentation, retraction, and a loss in processes were observed. Paclitaxel showed adverse effects on primary DRG non-neuronal cells, which might have adverse functional consequences on sensory neurons of the DRG, asking for consideration in the management of peripheral neuropathy.
Keywords: BrdU assay; DRG non-neuronal cells; LDH assay; MTT assay; paclitaxel; peripheral neuropathy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Palmitoylethanolamide Mitigates Paclitaxel Toxicity in Primary Dorsal Root Ganglion Neurons.Biomolecules. 2022 Dec 14;12(12):1873. doi: 10.3390/biom12121873. Biomolecules. 2022. PMID: 36551301 Free PMC article.
-
CX3CL1-mediated macrophage activation contributed to paclitaxel-induced DRG neuronal apoptosis and painful peripheral neuropathy.Brain Behav Immun. 2014 Aug;40:155-65. doi: 10.1016/j.bbi.2014.03.014. Epub 2014 Mar 26. Brain Behav Immun. 2014. PMID: 24681252
-
DRG Voltage-Gated Sodium Channel 1.7 Is Upregulated in Paclitaxel-Induced Neuropathy in Rats and in Humans with Neuropathic Pain.J Neurosci. 2018 Jan 31;38(5):1124-1136. doi: 10.1523/JNEUROSCI.0899-17.2017. Epub 2017 Dec 18. J Neurosci. 2018. PMID: 29255002 Free PMC article.
-
[Role of Transient Receptor Potential Channels in Paclitaxel- and Oxaliplatin-induced Peripheral Neuropathy].Yakugaku Zasshi. 2016;136(2):287-96. doi: 10.1248/yakushi.15-00214. Yakugaku Zasshi. 2016. PMID: 26831807 Review. Japanese.
-
Modelling the dorsal root ganglia using human pluripotent stem cells: A platform to study peripheral neuropathies.Int J Biochem Cell Biol. 2018 Jul;100:61-68. doi: 10.1016/j.biocel.2018.05.005. Epub 2018 Jun 1. Int J Biochem Cell Biol. 2018. PMID: 29772357 Review.
Cited by
-
The potential neuroprotective effects of cannabinoids against paclitaxel-induced peripheral neuropathy: in vitro study on neurite outgrowth.Front Pharmacol. 2024 Jun 12;15:1395951. doi: 10.3389/fphar.2024.1395951. eCollection 2024. Front Pharmacol. 2024. PMID: 38933665 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

