Sodium Propionate Relieves LPS-Induced Inflammation by Suppressing the NF-ĸB and MAPK Signaling Pathways in Rumen Epithelial Cells of Holstein Cows
- PMID: 37505707
- PMCID: PMC10467098
- DOI: 10.3390/toxins15070438
Sodium Propionate Relieves LPS-Induced Inflammation by Suppressing the NF-ĸB and MAPK Signaling Pathways in Rumen Epithelial Cells of Holstein Cows
Abstract
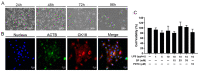
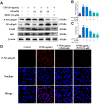
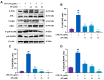
Subacute ruminal acidosis (SARA) is a prevalent disease in intensive dairy farming, and the rumen environment of diseased cows acidifies, leading to the rupture of gram-negative bacteria to release lipopolysaccharide (LPS). LPS can cause rumentitis and other complications, such as liver abscess, mastitis and laminitis. Propionate, commonly used in the dairy industry as a feed additive, has anti-inflammatory effects, but its mechanism is unclear. This study aims to investigate whether sodium propionate (SP) reduces LPS-induced inflammation in rumen epithelial cells (RECs) and the underlying mechanism. RECs were stimulated with different time (0, 1, 3, 6, 9, 18 h) and different concentrations of LPS (0, 1, 5, 10 μg/mL) to establish an inflammation model. Then, RECs were treated with SP (15, 25, 35 mM) or 10 μM PDTC in advance and stimulated by LPS for the assessment. The results showed that LPS (6h and 10 μg/mL) could stimulate the phosphorylation of NF-κB p65, IκB, JNK, ERK and p38 MAPK through TLR4, and increase the release of TNF-α, IL-1β and IL-6. SP (35 mM) can reduce the expression of cytokines by effectively inhibiting the NF-κB and MAPK inflammatory pathways. This study confirmed that SP inhibited LPS-induced inflammatory responses through NF-κB and MAPK in RECs, providing potential therapeutic targets and drugs for the prevention and treatment of SARA.
Keywords: inflammation; lipopolysaccharide; rumen epithelial cells; sodium propionate; subacute ruminal acidosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Castillo-Lopez E., Rivera-Chacon R., Ricci S., Petri R.M., Reisinger N., Zebeli Q. Short-term screening of multiple phytogenic compounds for their potential to modulate chewing behavior, ruminal fermentation profile, and pH in cattle fed grain-rich diets. J. Dairy Sci. 2021;104:4271–4289. doi: 10.3168/jds.2020-19521. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

