Increased Binding of von Willebrand Factor to Sub-Endothelial Collagen May Facilitate Thrombotic Events Complicating Bothrops lanceolatus Envenomation in Humans
- PMID: 37505710
- PMCID: PMC10467054
- DOI: 10.3390/toxins15070441
Increased Binding of von Willebrand Factor to Sub-Endothelial Collagen May Facilitate Thrombotic Events Complicating Bothrops lanceolatus Envenomation in Humans
Abstract
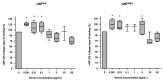
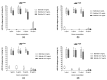
Consumption coagulopathy and hemorrhagic syndrome exacerbated by blood anticoagulability remain the most important causes of lethality associated with Bothrops snake envenomation. Bothrops venom also engages platelet aggregation on the injured endothelium via von Willebrand factor (vWF) interactions. Besides platelet aggregation, some Bothrops venom toxins may induce qualitative thrombopathy, which has been in part related to the inhibition of vWF activation. We tested whether B. lanceolatus venom impaired vWF to collagen(s) binding (vWF:CB) activity. Experiments were performed with B. lanceolatus crude venom, in the presence or absence of Bothrofav, a monospecific B. lanceolatus antivenom. Venom of B. lanceolatus fully inhibited vWF to collagen type I and III binding, suggesting venom interactions with the vWF A3 domain. In contrast, B. lanceolatus venom increased vWF to collagen type VI binding, suggesting the enhancement of vWF binding to collagen at the vWF A1 domain. Hence, B. lanceolatus venom exhibited contrasting in vitro effects in terms of the adhesive properties of vWF to collagen. On the other hand, the antivenom Bothrofav reversed the inhibitory effects of B. lanceolatus venom on vWF collagen binding activity. In light of the respective distribution of collagen type III and collagen type VI in perivascular connective tissue and the sub-endothelium, a putative association between an increase in vWF:CB activity for collagen type VI and the onset of thrombotic events in human B. lanceolatus envenomation might be considered.
Keywords: B. lanceolatus; Bothrops snake; collagen binding activity; envenomation; von Willebrand factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
The Contrasting Effects of Bothrops lanceolatus and Bothrops atrox Venom on Procoagulant Activity and Thrombus Stability under Blood Flow Conditions.Toxins (Basel). 2024 Sep 18;16(9):400. doi: 10.3390/toxins16090400. Toxins (Basel). 2024. PMID: 39330858 Free PMC article.
-
Enzymatic and Pro-Inflammatory Activities of Bothrops lanceolatus Venom: Relevance for Envenomation.Toxins (Basel). 2017 Aug 7;9(8):244. doi: 10.3390/toxins9080244. Toxins (Basel). 2017. PMID: 28783135 Free PMC article.
-
Involvement of von Willebrand factor and botrocetin in the thrombocytopenia induced by Bothrops jararaca snake venom.PLoS Negl Trop Dis. 2021 Sep 3;15(9):e0009715. doi: 10.1371/journal.pntd.0009715. eCollection 2021 Sep. PLoS Negl Trop Dis. 2021. PMID: 34478462 Free PMC article.
-
Structure and function of snake venom toxins interacting with human von Willebrand factor.Toxicon. 2005 Jun 15;45(8):1075-87. doi: 10.1016/j.toxicon.2005.02.023. Epub 2005 Apr 22. Toxicon. 2005. PMID: 15922776 Review.
-
Snake Bites by Bothrops lanceolatus in Martinique.Med Sante Trop. 2018 Feb 1;28(1):37-43. doi: 10.1684/mst.2018.0760. Med Sante Trop. 2018. PMID: 29616641 Review. English.
Cited by
-
Plasma von Willebrand factor levels in patients with cancer: A meta‑analysis.Oncol Lett. 2024 Jun 26;28(2):399. doi: 10.3892/ol.2024.14532. eCollection 2024 Aug. Oncol Lett. 2024. PMID: 38979552 Free PMC article.
-
The Contrasting Effects of Bothrops lanceolatus and Bothrops atrox Venom on Procoagulant Activity and Thrombus Stability under Blood Flow Conditions.Toxins (Basel). 2024 Sep 18;16(9):400. doi: 10.3390/toxins16090400. Toxins (Basel). 2024. PMID: 39330858 Free PMC article.
-
A murine experimental model of the pulmonary thrombotic effect induced by the venom of the snake Bothrops lanceolatus.PLoS Negl Trop Dis. 2024 Oct 2;18(10):e0012335. doi: 10.1371/journal.pntd.0012335. eCollection 2024 Oct. PLoS Negl Trop Dis. 2024. PMID: 39356725 Free PMC article.
References
-
- de Queiroz M.R., de Sousa B.B., da Cunha Pereira D.F., Mamede C.C.N., Matias M.S., de Morais N.C.G., de Oliveira Costa J., de Oliveira F. The role of platelets in hemostasis and the effects of snake venom toxins on platelet function. Toxicon. 2017;133:33–47. doi: 10.1016/j.toxicon.2017.04.013. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

