Emodin, an Emerging Mycotoxin, Induces Endoplasmic Reticulum Stress-Related Hepatotoxicity through IRE1α-XBP1 Axis in HepG2 Cells
- PMID: 37505724
- PMCID: PMC10467057
- DOI: 10.3390/toxins15070455
Emodin, an Emerging Mycotoxin, Induces Endoplasmic Reticulum Stress-Related Hepatotoxicity through IRE1α-XBP1 Axis in HepG2 Cells
Abstract
Emodin, an emerging mycotoxin, is known to be hepatotoxic, but its mechanism remains unclear. We hypothesized that emodin could induce endoplasmic reticulum (ER) stress through the inositol-requiring enzyme 1 alpha (IRE1α)-X-box-binding protein 1 (XBP1) pathway and apoptosis, which are closely correlated and contribute to hepatotoxicity. To test this hypothesis, a novel IRE1α inhibitor, STF-083010, was used. An MTT assay was used to evaluate metabolic activity, and quantitative PCR and western blotting were used to investigate the gene and protein expression of ER stress or apoptosis-related markers. Apoptosis was evaluated with flow cytometry. Results showed that emodin induced cytotoxicity in a dose-dependent manner in HepG2 cells and upregulated the expression of binding immunoglobulin protein (BiP), C/EBP homologous protein (CHOP), IRE1α, spliced XBP1, the B-cell lymphoma 2 (Bcl-2)-associated X protein (Bax)/Bcl-2 ratio, and cleaved caspase-3. Cotreatment with emodin and STF-083010 led to the downregulation of BiP and upregulation of CHOP, the Bax/Bcl-2 ratio, and cleaved caspase-3 compared with single treatment with emodin. Furthermore, the apoptosis rate was increased in a dose-dependent manner with emodin treatment. Thus, emodin induced ER stress in HepG2 cells by activating the IRE1α-XBP1 axis and induced apoptosis, indicating that emodin can cause hepatotoxicity.
Keywords: ER stress; HepG2 cells; IRE1α−XBP1; apoptosis; emodin; hepatotoxicity; mycotoxin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
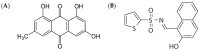






References
-
- Sulyok M., Krska R., Schuhmacher R. Application of an LC–MS/MS based multi-mycotoxin method for the semi-quantitative determination of mycotoxins occurring in different types of food infected by moulds. Food Chem. 2010;119:408–416. doi: 10.1016/j.foodchem.2009.07.042. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

