Fecal Calprotectin Concentrations in Cats with Chronic Enteropathies
- PMID: 37505825
- PMCID: PMC10385529
- DOI: 10.3390/vetsci10070419
Fecal Calprotectin Concentrations in Cats with Chronic Enteropathies
Abstract
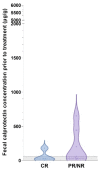
Diagnosis of feline chronic inflammatory enteropathies (CIE) and the differentiation from small cell intestinal lymphoma (SCL) can be challenging. Intestinally expressed calprotectin (S100A8/A9 protein complex) appears to be part of the complex pathogenesis of feline chronic enteropathies (FCE). Fecal calprotectin is a non-invasive biomarker for intestinal inflammation in humans and dogs but has not yet been evaluated in cats. We hypothesized that fecal calprotectin (fCal) concentrations are increased in FCE, correlate with clinical and/or histologic disease severity, and distinguish cases of CIE from SCL. This case-control study included fecal samples and patient data from cats with CIE (n = 34), SCL (n = 17), other gastrointestinal (GI) diseases (n = 16), and cats with no clinical signs of GI disease (n = 32). fCal concentrations were measured using the immunoturbidimetric fCal turbo assay (Bühlmann Laboratories). Compared to healthy cats, fCal concentrations were significantly increased in CIE, SCL, and other diseases (all p < 0.0001), but were not different between these three groups (all p > 0.05), or between cats with extra-GI diseases and healthy controls. These findings suggest that fCal may have utility as a clinical biomarker for FCE but not for intestinal disease differentiation. It further supports the role of calprotectin in the pathogenesis of the spectrum of FCE, which includes CIE and SCL.
Keywords: biomarker; feline chronic enteropathy; food-responsive enteropathy; gastrointestinal diet; hydrolyzed diet; inflammatory bowel disease; small cell lymphoma; steroid-responsive enteropathy.
Conflict of interest statement
Our study was performed with the generous support of Bühlmann Laboratories, Schönenbuch, BL, Switzerland. The company provided analyzer reagents, expertise in the application, and assay-specific materials. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Washabau R.J., Day M.J., Willard M.D., Hall E.J., Jergens A.E., Mansell J., Minami T., Bilzer T.W. Endoscopic, Biopsy, and Histopathologic Guidelines for the Evaluation of Gastrointestinal Inflammation in Companion Animals. J. Vet. Intern. Med. 2010;24:10–26. doi: 10.1111/J.1939-1676.2009.0443.X. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

