Inorganic phosphate exporter heterozygosity in mice leads to brain vascular calcification, microangiopathy, and microgliosis
- PMID: 37505935
- PMCID: PMC10580014
- DOI: 10.1111/bpa.13189
Inorganic phosphate exporter heterozygosity in mice leads to brain vascular calcification, microangiopathy, and microgliosis
Abstract
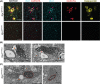
Calcification of the cerebral microvessels in the basal ganglia in the absence of systemic calcium and phosphate imbalance is a hallmark of primary familial brain calcification (PFBC), a rare neurodegenerative disorder. Mutation in genes encoding for sodium-dependent phosphate transporter 2 (SLC20A2), xenotropic and polytropic retrovirus receptor 1 (XPR1), platelet-derived growth factor B (PDGFB), platelet-derived growth factor receptor beta (PDGFRB), myogenesis regulating glycosidase (MYORG), and junctional adhesion molecule 2 (JAM2) are known to cause PFBC. Loss-of-function mutations in XPR1, the only known inorganic phosphate exporter in metazoans, causing dominantly inherited PFBC was first reported in 2015 but until now no studies in the brain have addressed whether loss of one functional allele leads to pathological alterations in mice, a commonly used organism to model human diseases. Here we show that mice heterozygous for Xpr1 (Xpr1WT/lacZ ) present with reduced inorganic phosphate levels in the cerebrospinal fluid and age- and sex-dependent growth of vascular calcifications in the thalamus. Vascular calcifications are surrounded by vascular basement membrane and are located at arterioles in the smooth muscle layer. Similar to previously characterized PFBC mouse models, vascular calcifications in Xpr1WT/lacZ mice contain bone matrix proteins and are surrounded by reactive astrocytes and microglia. However, microglial activation is not confined to calcified vessels but shows a widespread presence. In addition to vascular calcifications, we observed vessel tortuosity and transmission electron microscopy analysis revealed microangiopathy-endothelial swelling, phenotypic alterations in vascular smooth muscle cells, and thickening of the basement membrane.
Keywords: acoustic startle response; dark microglia; inflammation; retina; scanning electron microscopy; sexual dimorphism.
© 2023 The Authors. Brain Pathology published by John Wiley & Sons Ltd on behalf of International Society of Neuropathology.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

