Synthesis of Enantiomerically Pure Bambus[6]urils Utilizing Orthogonal Protection of Glycolurils
- PMID: 37505936
- PMCID: PMC10442914
- DOI: 10.1021/acs.joc.3c00667
Synthesis of Enantiomerically Pure Bambus[6]urils Utilizing Orthogonal Protection of Glycolurils
Abstract
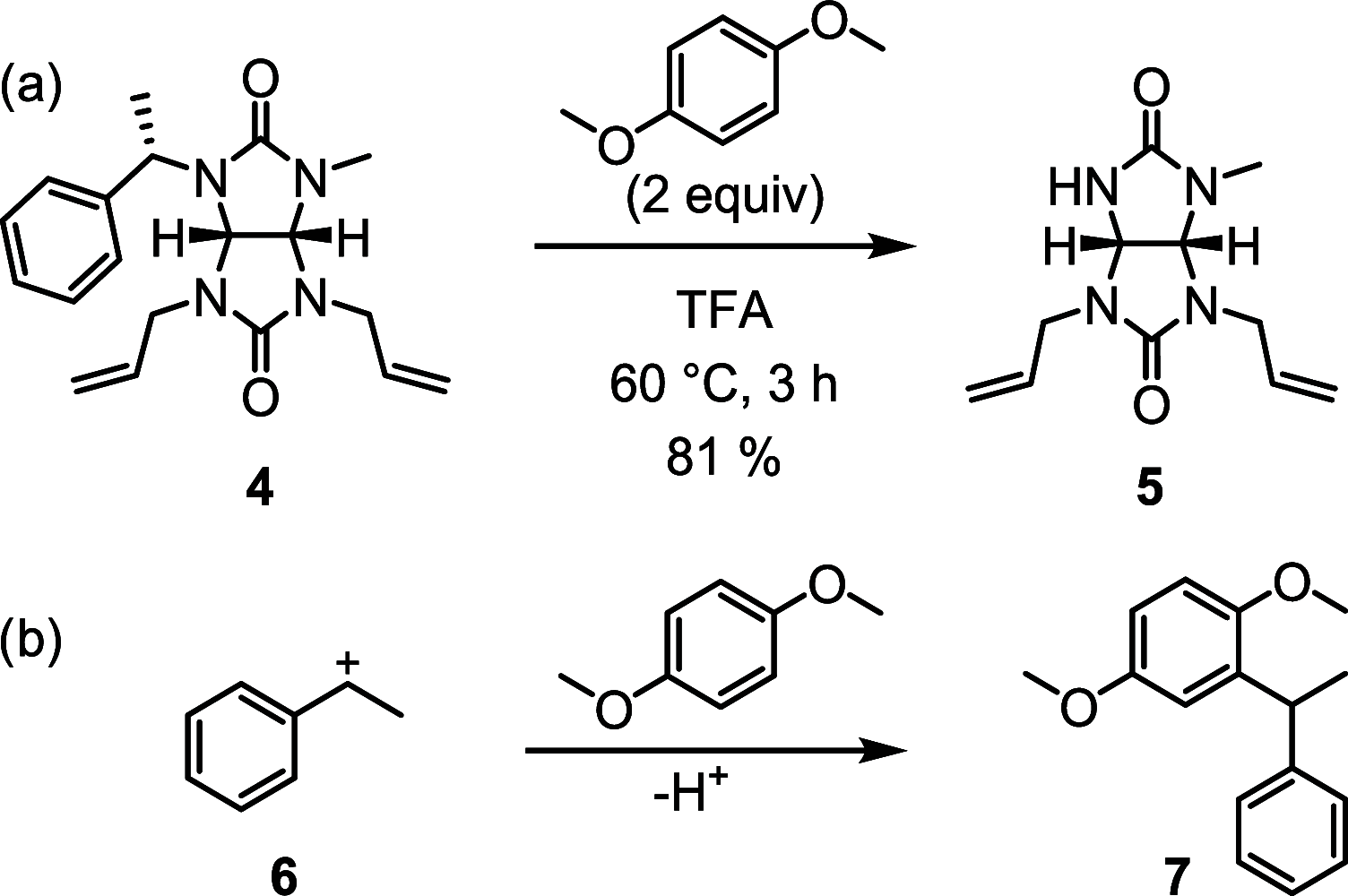
A general strategy for the synthesis of 2N,4N'-disubstituted glycoluril enantiomers on a multigram scale using orthogonal protection is reported. The use of these glycolurils is demonstrated in the synthesis of enantiomerically pure bambus[6]uril macrocycles. Moreover, the deprotection of (S)-1-phenylethyl substituents on the macrocycle was achieved, opening access to various chiral bambus[6]urils via post-macrocyclization modification strategy.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Merging Bambus[6]uril and Biotin[6]uril into an Enantiomerically Pure Monofunctionalized Hybrid Macrocycle.Org Lett. 2024 Jan 12;26(1):106-109. doi: 10.1021/acs.orglett.3c03715. Epub 2023 Dec 28. Org Lett. 2024. PMID: 38153981 Free PMC article.
-
Inherently Chiral Bambus[4]urils.J Org Chem. 2020 Jul 17;85(14):9190-9200. doi: 10.1021/acs.joc.0c01174. Epub 2020 Jun 25. J Org Chem. 2020. PMID: 32521998
-
Bambus[n]urils: a new family of macrocyclic anion receptors.Org Lett. 2011 Aug 5;13(15):4000-3. doi: 10.1021/ol201515c. Epub 2011 Jun 28. Org Lett. 2011. PMID: 21707115
-
Development of Novel Composite Biocompatible Materials by Surface Modification of Porous Inorganic Compounds Using Bambus[6]Uril.Materials (Basel). 2023 Nov 21;16(23):7257. doi: 10.3390/ma16237257. Materials (Basel). 2023. PMID: 38068001 Free PMC article.
-
Macrocyclization via C-H functionalization: a new paradigm in macrocycle synthesis.Org Biomol Chem. 2020 Mar 11;18(10):1851-1876. doi: 10.1039/c9ob02765c. Org Biomol Chem. 2020. PMID: 32101232 Review.
Cited by
-
Anion Transport by Bambusuril-Bile Acid Conjugates: Drastic Effect of the Cholesterol Content.Angew Chem Int Ed Engl. 2025 Feb 24;64(9):e202424754. doi: 10.1002/anie.202424754. Epub 2025 Jan 21. Angew Chem Int Ed Engl. 2025. PMID: 39791967 Free PMC article.
References
-
- Liang X.; Liang W.; Jin P.; Wang H.; Wu W.; Yang C. Advances in Chirality Sensing with Macrocyclic Molecules. Chemosensors 2021, 9, 279.10.3390/chemosensors9100279. - DOI
-
- Rowan A. E.; Elemans J. A. A. W.; Nolte R. J. M. Molecular and Supramolecular Objects from Glycoluril. Acc. Chem. Res. 1999, 32, 995–1006. 10.1021/ar9702684. - DOI
LinkOut - more resources
Full Text Sources

