Plasma proteomic profiling identifies CD33 as a marker of HIV control in natural infection and after therapeutic vaccination
- PMID: 37506557
- PMCID: PMC10410179
- DOI: 10.1016/j.ebiom.2023.104732
Plasma proteomic profiling identifies CD33 as a marker of HIV control in natural infection and after therapeutic vaccination
Abstract
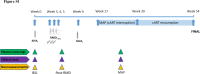
Background: Biomarkers predicting the outcome of HIV-1 virus control in natural infection and after therapeutic interventions in HIV-1 cure trials remain poorly defined. The BCN02 trial (NCT02616874), combined a T-cell vaccine with romidepsin (RMD), a cancer-drug that was used to promote HIV-1 latency reversal and which has also been shown to have beneficial effects on neurofunction. We conducted longitudinal plasma proteomics analyses in trial participants to define biomarkers associated with virus control during monitored antiretroviral pause (MAP) and to identify novel therapeutic targets that can improve future cure strategies.
Methods: BCN02 was a phase I, open-label, single-arm clinical trial in early-treated, HIV infected individuals. Longitudinal plasma proteomes were analyzed in 11 BCN02 participants, including 8 participants that showed a rapid HIV-1 plasma rebound during a monitored antiretroviral pause (MAP-NC, 'non-controllers') and 3 that remained off ART with sustained plasma viremia <2000 copies/ml (MAP-C, 'controllers'). Inflammatory and neurological proteomes in plasma were evaluated and integration data analysis (viral and neurocognitive parameters) was performed. Validation studies were conducted in a cohort of untreated HIV-1+ individuals (n = 96) and in vitro viral replication assays using an anti-CD33 antibody were used for functional validation.
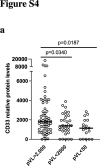
Findings: Inflammatory plasma proteomes in BCN02 participants showed marked longitudinal alterations. Strong proteome differences were also observed between MAP-C and MAP-NC, including in baseline timepoints. CD33/Siglec-3 was the unique plasma marker with the ability to discriminate between MAPC-C and MAP-NC at all study timepoints and showed positive correlations with viral parameters. Analyses in an untreated cohort of PLWH confirmed the positive correlation between viral parameters and CD33 plasma levels, as well as PBMC gene expression. Finally, adding an anti-CD33 antibody to in vitro virus cultures significantly reduced HIV-1 replication and proviral levels in T cells and macrophages.
Interpretation: This study indicates that CD33/Siglec-3 may serve as a predictor of HIV-1 control and as potential therapeutic tool to improve future cure strategies.
Funding: Spanish Science and Innovation Ministry (SAF2017-89726-R and PID2020-119710RB-I00), NIH (P01-AI131568), European Commission (GA101057548) and a Grifols research agreement.
Keywords: Control of HIV-1 infection; Inflammation; Kick and kill HIV cure strategy; Neurological function; Plasma proteomics; Siglec-3/CD33.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests BM is a consultant of AELIX THERAPEUTICS, SL outside the submitted work. CB is co-founder, chief science officer and shareholder of AELIX THERAPEUTICS. TH is a coinventor of the HIVconsv immunogen. All other authors declare that they have no competing interests.
Figures




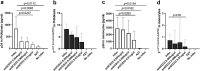

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

