SUZ domain-containing proteins have multiple effects on nonsense-mediated decay target transcripts
- PMID: 37507022
- PMCID: PMC10470013
- DOI: 10.1016/j.jbc.2023.105095
SUZ domain-containing proteins have multiple effects on nonsense-mediated decay target transcripts
Abstract
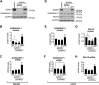
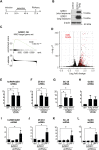
Many transcripts are targeted by nonsense-mediated decay (NMD), leading to their degradation and the inhibition of their translation. We found that the protein SUZ domain-containing protein 1 (SZRD1) interacts with the key NMD factor up-frameshift 1. When recruited to NMD-sensitive reporter gene transcripts, SZRD1 increased protein production, at least in part, by relieving translational inhibition. The conserved SUZ domain in SZRD1 was required for this effect. The SUZ domain is present in only three other human proteins besides SZRD1: R3H domain-containing protein 1 and 2 (R3HDM1, R3HDM2) and cAMP-regulated phosphoprotein 21 (ARPP21). We found that ARPP21, similarly to SZRD1, can increase protein production from NMD-sensitive reporter transcripts in an SUZ domain-dependent manner. This indicated that the SUZ domain-containing proteins could prevent translational inhibition of transcripts targeted by NMD. Consistent with the idea that SZRD1 mainly prevents translational inhibition, we did not observe a systematic decrease in the abundance of NMD targets when we knocked down SZRD1. Surprisingly, knockdown of SZRD1 in two different cell lines led to reduced levels of the NMD component UPF3B, which was accompanied by increased levels in a subset of NMD targets. This suggests that SZRD1 is required to maintain normal UPF3B levels and indicates that the effect of SZRD1 on NMD targets is not limited to a relief from translational inhibition. Overall, our study reveals that human SUZ domain-containing proteins play a complex role in regulating protein output from transcripts targeted by NMD.
Keywords: SUZ domain; SUZ-C domain; nonsense-mediated decay; protein domain; translation.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures





References
-
- Leeds P., Peltz S.W., Jacobson A., Culbertson M.R. The product of the yeast UPF1 gene is required for rapid turnover of mRNAs containing a premature translational termination codon. Genes Dev. 1991;5:2303–2314. - PubMed
-
- Chang J.C., Temple G.F., Trecartin R.F., Kan Y.W. Suppression of the nonsense mutation in homozygous beta 0 thalassaemia. Nature. 1979;281:602–603. - PubMed
-
- Maquat L.E., Kinniburgh A.J., Rachmilewitz E.A., Ross J. Unstable beta-globin mRNA in mRNA-deficient beta o thalassemia. Cell. 1981;27:543–553. - PubMed
-
- Buhler M., Steiner S., Mohn F., Paillusson A., Muhlemann O. EJC-independent degradation of nonsense immunoglobulin-mu mRNA depends on 3' UTR length. Nat. Struct. Mol. Biol. 2006;13:462–464. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

