Safety and immunogenicity of a SARS-CoV-2 Gamma variant RBD-based protein adjuvanted vaccine used as booster in healthy adults
- PMID: 37507392
- PMCID: PMC10382514
- DOI: 10.1038/s41467-023-40272-3
Safety and immunogenicity of a SARS-CoV-2 Gamma variant RBD-based protein adjuvanted vaccine used as booster in healthy adults
Abstract
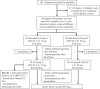
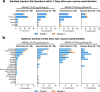
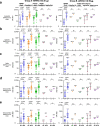
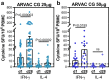
A Gamma Variant RBD-based aluminum hydroxide adjuvanted vaccine called ARVAC CG was selected for a first in human clinical trial. Healthy male and female participants (18-55 years old) with a complete COVID-19-primary vaccine scheme were assigned to receive two intramuscular doses of either a low-dose or a high-dose of ARVAC CG. The primary endpoint was safety. The secondary objective was humoral immunogenicity. Cellular immune responses were studied as an exploratory objective. The trial was prospectively registered in PRIISA.BA (Registration Code 6564) and ANMAT and retrospectively registered in ClinicalTrials.gov (NCT05656508). Samples from participants of a surveillance strategy implemented by the Ministry of Health of the Province of Buenos Aires that were boosted with BNT162b2 were also analyzed to compare with the booster effect of ARVAC CG. ARVAC CG exhibits a satisfactory safety profile, a robust and broad booster response of neutralizing antibodies against the Ancestral strain of SARS-CoV-2 and the Gamma, Delta, Omicron BA.1 and Omicron BA.5 variants of concern and a booster effect on T cell immunity in individuals previously immunized with different COVID-19 vaccine platforms.
© 2023. The Author(s).
Conflict of interest statement
J.M.R., L.P.C. R&D and CMC Group, F.M.O., J.C.V. are employees of Laboratorio Pablo Cassará S.R.L., which developed the vaccine and funded the trial. M.L. and J.F. are external consultants and received honoraria from Laboratorio Pablo Cassará S.R.L. All other authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

