Serum granulosa cell-derived TNF-α promotes inflammation and apoptosis of renal tubular cells and PCOS-related kidney injury through NF-κB signaling
- PMID: 37507430
- PMCID: PMC10692080
- DOI: 10.1038/s41401-023-01128-0
Serum granulosa cell-derived TNF-α promotes inflammation and apoptosis of renal tubular cells and PCOS-related kidney injury through NF-κB signaling
Abstract
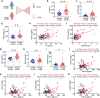
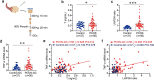
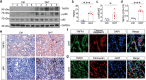
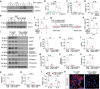
Polycystic ovary syndrome (PCOS) is a disorder with endocrinal and metabolic problems in reproductive aged women. Evidence shows that PCOS is in a high prone trend to develop kidney diseases. In this study, we investigated the mediators responsible for PCOS-related kidney injury. We found that tumor necrosis factor (TNF-α) levels were significantly increased in serum and primary cultured granulosa cells (GCs) from PCOS patients. Serum TNF-α levels were positively correlated with serum testosterone and luteinizing hormone (LH)/follicle-stimulating hormone (FSH) ratio, suggesting its positive role in the severity of PCOS. Serum TNF-α levels were also positively correlated with the levels of urinary KapU, LamU, α1-MU and β2-MU, the markers for renal tubular cell-derived proteinuria. We established a PCOS mouse model by resection of the right kidney, followed by daily administration of dihydrotestosterone (DHT, 27.5 μg, i.p.) from D7 for 90 days. We found that TNF-α levels were significantly increased in the ovary and serum of the mice, accompanied by increased renal tubular cell apoptosis, inflammation and fibrosis in kidneys. Furthermore, the receptor of TNF-α, tumor necrosis factor receptor 1 (TNFR1), was significantly upregulated in renal tubular cells. We treated human ovarian granulosa-like tumor cells (KGN) with DHT (1 μg/ml) in vitro, the conditioned medium derived from the granulosa cell culture greatly accelerated apoptotic injury in human proximal tubular epithelial cells (HKC-8), which was blocked after knockdown of TNF-α in KGN cells. Furthermore, knockdown of TNFR1 in renal tubular epithelial cells greatly ameliorated cell injury induced by granulosa cell-derived conditioned medium. These results suggest that serum TNF-α plays a key role in mediating inflammation and apoptosis in renal tubular cells associated with PCOS-related kidney injury.
Keywords: TNF-α; TNFR1; apoptosis; inflammation; kidney injury; polycystic ovary syndrome.
© 2023. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-sponsored 3rd PCOS consensus workshop group. Fertil Steril. 2012;97:28–38.e25. doi: 10.1016/j.fertnstert.2011.09.024. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

